Pathogenic de novo variants in PPP2R5C cause a neurodevelopmental disorder within the Houge-Janssens syndrome spectrum
- PMID: 39978342
- PMCID: PMC11947181
- DOI: 10.1016/j.ajhg.2025.01.021
Pathogenic de novo variants in PPP2R5C cause a neurodevelopmental disorder within the Houge-Janssens syndrome spectrum
Abstract
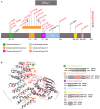
Pathogenic variants resulting in protein phosphatase 2A (PP2A) dysfunction result in mild to severe neurodevelopmental delay. PP2A is a trimer of a catalytic (C) subunit, scaffolding (A) subunit, and substrate binding/regulatory (B) subunit, encoded by 19 different genes. De novo missense variants in PPP2R5D (B56δ) or PPP2R1A (Aα) and de novo missense and loss-of-function variants in PPP2CA (Cα) lead to syndromes with overlapping phenotypic features, known as Houge-Janssens syndrome (HJS) types 1, 2, and 3, respectively. Here, we describe an additional condition in the HJS spectrum in 26 individuals with variants in PPP2R5C, encoding the regulatory B56γ subunit. Most changes were de novo and of the missense type. The clinical features were well within the HJS spectrum with strongest resemblance to HJS type 1, caused by B56δ variants. Common features were neurodevelopmental delay and hypotonia, with a high risk of epilepsy, behavioral problems, and mildly dysmorphic facial features. Head circumferences were above average or macrocephalic. The degree of intellectual disability was, on average, milder than in other HJS types. All variants affected either substrate binding (2/19), C-subunit binding (2/19), or both (15/19). Five variants were recurrent. Catalytic activity of the phosphatase was variably affected by the variants. Of note, PPP2R5C total loss-of-function variants could be inherited from a non-symptomatic parent. This implies that a dominant-negative mechanism on substrate dephosphorylation or general PP2A function is the most likely pathogenic mechanism.
Keywords: PP2A; PPP2R5C; PPP2R5D; autism; developmental delay; epilepsy; intellectual disability; macrocephaly; neurodevelopmental disorder; protein phosphatase 2A.
Copyright © 2025 American Society of Human Genetics. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures






References
-
- Houge G., Haesen D., Vissers L.E.L.M., Mehta S., Parker M.J., Wright M., Vogt J., McKee S., Tolmie J.L., Cordeiro N., et al. B56δ-related protein phosphatase 2A dysfunction identified in patients with intellectual disability. J. Clin. Investig. 2015;125:3051–3062. doi: 10.1172/JCI79860. - DOI - PMC - PubMed
-
- Loveday C., Tatton-Brown K., Clarke M., Westwood I., Renwick A., Ramsay E., Nemeth A., Campbell J., Joss S., Gardner M., et al. Mutations in the PP2A regulatory subunit B family genes PPP2R5B, PPP2R5C and PPP2R5D cause human overgrowth. Hum. Mol. Genet. 2015;24:4775–4779. doi: 10.1093/hmg/ddv182. - DOI - PMC - PubMed
-
- Reynhout S., Jansen S., Haesen D., van Belle S., de Munnik S.A., Bongers E.M.H.F., Schieving J.H., Marcelis C., Amiel J., Rio M., et al. De novo mutations affecting the catalytic Calpha subunit of PP2A, PPP2CA, cause syndromic intellectual disability resembling other PP2A-related neurodevelopmental disorders. Am. J. Hum. Genet. 2019;104:139–156. doi: 10.1016/j.ajhg.2018.12.002. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

