Post-Hospitalisation COVID-19 Rehabilitation (PHOSP-R): a randomised controlled trial of exercise-based rehabilitation
- PMID: 39978856
- PMCID: PMC12095904
- DOI: 10.1183/13993003.02152-2024
Post-Hospitalisation COVID-19 Rehabilitation (PHOSP-R): a randomised controlled trial of exercise-based rehabilitation
Abstract
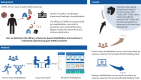
Objective: Post-COVID syndrome involves prolonged symptoms with multisystem and functional impairment lasting ≥12 weeks after acute coronavirus disease 2019 (COVID-19). We aimed to determine the efficacy of exercise-based rehabilitation interventions, either face-to-face or remote, compared to usual care in individuals experiencing post-COVID syndrome following a hospitalisation with acute COVID-19.
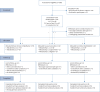
Design: This single-blind randomised controlled trial compared two exercise-based rehabilitation interventions (face-to-face or remote) to usual care in participants with post-COVID syndrome following a hospitalisation. The interventions were either a face-to-face or remote 8-week programme of individually prescribed exercise and education. The primary outcome was the change in Incremental Shuttle Walking Test (ISWT) following 8 weeks of intervention (either face-to-face or remote) compared to usual care. Other secondary outcomes were measured including health-related quality of life (HRQoL), and exploratory outcomes included lymphocyte immunotyping.
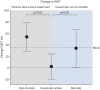
Results: 181 participants (55% male, mean±sd age 59±12 years, length of hospital stay 12±19 days) were randomised. There was an improvement in the ISWT distance following face-to-face rehabilitation (mean 52 m, 95% CI 19-85 m; p=0.002) and remote rehabilitation (mean 34 m, 95% CI 1-66 m; p=0.047) compared to usual care alone. There were no differences between groups for HRQoL self-reported symptoms. Analysis of immune markers revealed significant increases in naïve and memory CD8+ T-cells following face-to-face rehabilitation versus usual care alone (p<0.001, n=31).
Conclusion: Exercise-based rehabilitation improved short-term exercise capacity in post-COVID syndrome following an acute hospitalisation and showed potential for beneficial immunomodulatory effects.
Copyright ©The authors 2025.
Conflict of interest statement
Conflict of interest: E. Daynes reports consulting fees from NMHI, honoraria from ClinicalPhysio, Neuroscience and Mental Health Institute (funding committee 2023), and leadership roles with the BTS as chair of the Pulmonary Rehabilitation Specialist Advisory Group (unpaid) and The Royal College of Physicians (paid). R.A. Evans reports funding from UKRI/MRC/NIHR, grants from Wolfson Foundation and Genentech/Roche, consulting fees for AstraZeneca/Evidera for long COVID, speaker fees for Boehringer and Moderna on the topic of long COVID, support for attending meetings from Chiesi, and leadership roles as Chair of ERS Group 01.02 Pulmonary Rehabilitation and Chronic Care, and Chair of ATS Pulmonary Rehabilitation Assembly. N.J. Greening reports consultancy fees for Genetech/Roche, honoraria from Chiesa, GSK, AstraZeneca and Pulmonx, and support for attending meetings from Chiesi and GSK. N.C. Bishop reports support for the present manuscript from NIHR-Leicester Biomedical Research Centre. M. Hamrouni reports support for the present manuscript from NIHR-Leicester Biomedical Research Centre. M. Roberts reports support for the present manuscript from NIHR. C.E. Bolton reports grants from NIHR, UKRI, Nottingham Hospitals Charity, UoN charitable gifts and Nottingham University Hospitals Trust. J.D. Chalmers reports grants and personal fees from AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Novartis and Insmed, personal fees from Chiesi, Zambon, Janssen and Grifols, and grants from Gilead Sciences, outside the submitted work. T. Chalder is part-funded by the National Institute for Health Research (NIHR) Biomedical Research Centre at South London and Maudsley NHS Foundation Trust, King's College London, and reports grants from Guy's and St Thomas’ Charity, NIHR and UKRI, is on the Expert Advisory Panel for Covid-19 Rapid Guidelines and the Scientific committee of BABCP, has received ad hoc payments for workshops carried out on long-term conditions, support for attending meetings from BABCP and EABCT, and is the author of several self-help books on chronic fatigue for which she has received royalties. A.B. Docherty reports funding from Wellcome in an unrelated fellowship (216606/Z/19/Z). L.P. Ho reports support from Roche/Genentech. A. Horsley reports support for the present manuscript from UKRI (MR/V027859/1), NIHR (COV0319) and NIHR Manchester BRC, and is Chair of NIHR Translational Research Collaboration (unpaid). J.K. Quint reports consulting fees for Evidera for advice on COVID-19 studies. B. Raman reports support for the present manuscript from Wellcome Trust (Wellcome Trust Career Development Award (302210/Z/23/Z)) and NIHR Oxford BRC. A. Singapuri reports support for the present manuscript from UKRI and NIHR (MR/V027859/1 and COV0319). L.V. Wain reports support for the present manuscript from UKRI, GSK/Asthma and Lung UK, and NIHR, grants from Orion Pharma, GSK, Genetech and AstraZeneca, consulting fees from Galapagos and Boehringer Ingelheim, support for attending meetings from Genentech, advisory board membership with Galapagos, and a leadership role as associate editor for the European Respiratory Journal. W.D-C. Man reports grants from NIHR and Small Business Research Initiative, and a leadership role as Honorary President of the Association for Respiratory Technology and Physiology. C. Brightling reports support for the present manuscript from PHOSP-COVID NIHR UKRI and NIHR Leicester BRC, and has received grants and consultancy fees from 4D Pharma, Areteia, AstraZeneca, Chiesi, Genentech, GlaxoSmithKline, Mologic, Novartis, Regeneron Pharmaceuticals, Roche and Sanofi. The remaining authors have no potential conflicts of interest to disclose.
Figures
Comment in
-
Rehabilitation for persons with long COVID beyond the recovery phase of SARS-CoV-2 infection.Eur Respir J. 2025 May 22;65(5):2500239. doi: 10.1183/13993003.00239-2025. Print 2025 May. Eur Respir J. 2025. PMID: 40404198 No abstract available.
References
-
- NHS England . Post-COVID syndrome (long COVID). Date last accessed: 20 April 2024. www.england.nhs.uk/coronavirus/post-covid-syndrome-long-covid/
-
- World Health Organization (WHO) . Post COVID-19 condition (long COVID). Date last accessed: 20 April 2024. Date last updated: 7 December 2022. www.who.int/europe/news-room/fact-sheets/item/post-covid-19-condition#:∼...
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
