Heart rate response and cardiovascular risk during obstructive sleep apnoea: an easy biomarker derived from pulse oximetry
- PMID: 39978860
- PMCID: PMC12056247
- DOI: 10.1183/13993003.01883-2024
Heart rate response and cardiovascular risk during obstructive sleep apnoea: an easy biomarker derived from pulse oximetry
Abstract
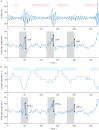
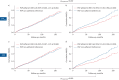
Background: Sleep apnoea-specific heart rate response (ΔHR) has been identified as a promising biomarker for stratifying cardiovascular (CV) risk and predicting positive airway pressure (PAP) benefit in obstructive sleep apnoea (OSA). However, the need for prior manual scoring of respiratory events potentially limits the accessibility and reproducibility of ΔHR. We aimed to evaluate the association of pulse rate response to oxygen desaturations automatically derived from pulse oximetry (ΔHRoxi) with CV risk in OSA.
Methods: ΔHRoxi and ΔHR were measured in OSA patients from the Institut de Recherche en Santé Respiratoire Pays de la Loire Sleep Cohort (PLSC; n=5002) and the HypnoLaus cohort (n=1307). The primary outcome was major adverse CV events (MACEs), a composite of mortality, stroke and cardiac diseases. Cox regression analyses were conducted to evaluate the association of ΔHRoxi and ΔHR, categorised into low, midrange and high categories, with MACEs.
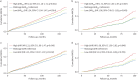
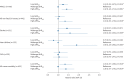
Results: MACEs occurred in 768 patients from PLSC and 87 patients from HypnoLaus (median follow-up 8.0 and 7.5 years, respectively). Multivariable Cox models showed that subjects with high ΔHRoxi (versus midrange) had higher risk of MACEs in PLSC (hazard ratio (HR) 1.42, 95% CI 1.18-1.71) and HypnoLaus (HR 1.72, 95% CI 1.03-2.87). Similar findings were observed for high ΔHR. Among 2718 patients from PLSC treated with PAP, the association of PAP adherence (PAP use ≥4 h·night-1 versus non-adherent) with MACEs was modified by baseline ΔHR and ΔHRoxi (pinteraction<0.05).
Conclusion: ΔHRoxi could constitute a reliable and easy to measure biomarker for stratifying CV risk and predicting CV benefit of PAP in OSA.
Copyright ©The authors 2025.
Conflict of interest statement
Conflict of interest: M. Blanchard reports grants from CIDELEC and IRSR-PL. G. Solelhac reports grants from Ligue Pulmonaire Vaudoise, consultancy fees from Wellnest Retreats, payment or honoraria for lectures, presentations, manuscript writing or educational events from Idorsia and Wellnest Retreats, and participation on a data safety monitoring board or advisory board with Idorsia. A. Sabil is an employee of SEFAM Medical. W. Trzepizur reports payment or honoraria for lectures, presentations, manuscript writing or educational events from AstraZeneca and Biprojet, and support for attending meetings from Asten Santé. A. Thomas reports receipt of equipment, materials, drugs, medical writing, gifts or other services from CIDLE. S. Bailly reports receipt of equipment, materials, drugs, medical writing, gifts or other services from CIDLE. A. Azarbarzin reports grants from the American Heart Association, NIH, American Academy of Sleep Medicine and Somnifix, consultancy fees from Somnifix, ZOLL Respicardia, Eli Lilly, Apnimed, Inspire and Cerebra, payment or honoraria for lectures, presentations, manuscript writing or educational events from Philips Respironics, ProSomnus and the British Royal Society of Medicine, patents for “System and method for endo-phenotyping and risk stratifying obstructive sleep apnoea” and “Method, non-transitory computer readable medium and apparatus for arousal intensity scoring”, and receipt of equipment from Philips Respironics. P. Vollenweider reports support for the present manuscript from GSK. J. Vaucher reports grants from the Swiss National Science Foundation (grant 32473B-182210), Leenaards Foundation, University of Fribourg, Swiss Society of Internal Medicine and AGLA Foundation. R. Heinzer reports support for the present study from GlaxoSmithKline, the Faculty of Biology and Medicine of Lausanne and the Swiss National Science Foundation (grants 33CSCO-122661, 33CS30-139468, 33CS30-148401, 33CS30_177535 and 3247730_204523), grants from Ligue Pulmonaire Vaudoise and Apnimed, consultancy fees from ResMed, Philips and Apnimed, and payment or honoraria for lectures, presentations, manuscript writing or educational events from ResMed, Jazz, Inspire, Bioprojet, Philips, Merck, Medtronic, Nestlé and Löwenstein. F. Gagnadoux reports support for the present study from ALTADIR and Institut Recherche en Santé Respiratoire des Pays de Loire (IRSR-PL), consultancy fees from ResMed, Inspire Medical, SEFAM, Bioprojet and Asten Santé, payment or honoraria for lectures, presentations, manuscript writing or educational events from ResMed, Inspire Medical, SEFAM, Bioprojet and CIDELEC, and support for attending meetings from ResMed, Inspire Medical, SEFAM and Bioprojet. The remaining authors have no potential conflicts of interest to disclose.
Figures

Similar articles
-
Positive Airway Pressure Adherence, Mortality, and Cardiovascular Events in Patients with Sleep Apnea.Am J Respir Crit Care Med. 2022 Dec 1;206(11):1393-1404. doi: 10.1164/rccm.202202-0366OC. Am J Respir Crit Care Med. 2022. PMID: 35816570
-
Pulse Wave Amplitude Drops Index: A Biomarker of Cardiovascular Risk in Obstructive Sleep Apnea.Am J Respir Crit Care Med. 2023 Jun 15;207(12):1620-1632. doi: 10.1164/rccm.202206-1223OC. Am J Respir Crit Care Med. 2023. PMID: 37017487 Free PMC article.
-
The Sleep Apnea-Specific Pulse-Rate Response Predicts Cardiovascular Morbidity and Mortality.Am J Respir Crit Care Med. 2021 Jun 15;203(12):1546-1555. doi: 10.1164/rccm.202010-3900OC. Am J Respir Crit Care Med. 2021. PMID: 33406013 Free PMC article.
-
Positive airway pressure therapy and all-cause and cardiovascular mortality in people with obstructive sleep apnoea: a systematic review and meta-analysis of randomised controlled trials and confounder-adjusted, non-randomised controlled studies.Lancet Respir Med. 2025 May;13(5):403-413. doi: 10.1016/S2213-2600(25)00002-5. Epub 2025 Mar 18. Lancet Respir Med. 2025. PMID: 40118084
-
Pressure modification or humidification for improving usage of continuous positive airway pressure machines in adults with obstructive sleep apnoea.Cochrane Database Syst Rev. 2019 Dec 2;12(12):CD003531. doi: 10.1002/14651858.CD003531.pub4. Cochrane Database Syst Rev. 2019. PMID: 31792939 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
