A long-term follow-up study of sotatercept for treatment of pulmonary arterial hypertension: interim results of SOTERIA
- PMID: 39978862
- PMCID: PMC12287605
- DOI: 10.1183/13993003.01435-2024
A long-term follow-up study of sotatercept for treatment of pulmonary arterial hypertension: interim results of SOTERIA
Abstract
Background: SOTERIA (ClinicalTrials.gov: NCT04796337) is an ongoing open-label study evaluating long-term safety, tolerability and efficacy of sotatercept in participants with pulmonary arterial hypertension (PAH).
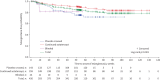
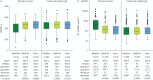
Methods: Eligible adults with PAH on stable background therapy who completed a prior sotatercept study without early discontinuation were enrolled. Participants received subcutaneous sotatercept (≤0.7 mg·kg-1 once every 21 days). Safety and tolerability (primary objective) were assessed by adverse events (AEs), vital signs and laboratory assessments. Efficacy (secondary objective) was assessed by 6-min walk distance (6MWD), N-terminal pro-brain natriuretic peptide (NT-proBNP) levels, World Health Organization (WHO) Functional Class, clinical worsening events and simplified French risk score (SFRS). The data cut-off date was 8 November 2023.
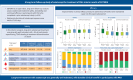
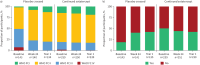
Results: Altogether, 426 participants were included in the analyses. Mean±sd duration of exposure to sotatercept and follow-up in SOTERIA was 448.6±172.93 days (range 21-923 days; 523 patient-years). Of 426 participants, 387 (90.8%) experienced AEs, 15 (3.5%) discontinued treatment, 129 (30.3%) had serious AEs and 11 (2.6%) had serious AEs related to treatment. There were 12 deaths (2.8%). Among AEs of interest, epistaxis (22.1%) and telangiectasia (16.9%) were the most frequently reported individual events. 22 (5.2%) participants had serious bleeding events, including two (0.5%) with serious bleeding leading to death (not related to treatment by investigator judgement). Improvements in 6MWD, NT-proBNP, WHO Functional Class and SFRS achieved from baseline of SOTERIA were largely maintained at 1 year, including in the placebo-crossed group.
Conclusion: Interim results of SOTERIA support the favourable benefit-risk of add-on sotatercept treatment in adults with PAH. Follow-up reports from this study will provide additional information on benefit-risk.
Copyright ©The authors 2025.
Conflict of interest statement
Conflicts of interest: I.R. Preston reports grants/research support from Janssen Global Services, LLC and United Therapeutics Corp.; steering committee work for Acceleron Pharma, Inc., Keros Therapeutics, Liquidia Corp. and United Therapeutics Corp.; consultancy for Janssen Global Services, LLC and Respira Therapeutics; and scientific advisory board work for Aerovate Therapeutics, Inc., Altavant Sciences and Gossamer Bio. I.R. Preston is currently at Lahey Hospital and Medical Center, Burlington, MA, USA. D. Badesch reports grants/research support from Acceleron Pharma, Inc. (clinical trial), Actelion Pharmaceuticals, Altavant Sciences, Arena Pharmaceuticals, Ikaria, Inc., Liquidia Corp., Merck & Co., Inc. and United Therapeutics Corp.; consultancy for Acceleron Pharma, Inc., Altavant Sciences, Arena Pharmaceuticals, Bayer, Liquidia, Merck & Co., Inc. and Pfizer Canada; data and safety monitoring board work for United Therapeutics Corp.; and stock/stock options in Johnson & Johnson Health Care Systems Inc. (spouse/partner). H-A. Ghofrani reports consultancy for Aerovate Therapeutics, Inc., Altavant Sciences, Bayer HealthCare, Gossamer Bio, Janssen Diagnostics, LLC, Merck & Co., Inc. and Pfizer Inc. J.S.R. Gibbs reports consultancy for Acceleron Pharma, Inc. and Merck & Co., Inc.; data and safety monitoring board work for Actelion Pharmaceuticals, Fundação Bial, Gossamer Bio, Keros Therapeutics and Merck & Co., Inc.; and end-point review committee work for Actelion Pharmaceuticals, Aerovate Therapeutics, Inc., Janssen Biotech, Inc., LG Chem, Pfizer Pharma GmbH and United Therapeutics Corp. M. Gomberg-Maitland reports consultancy for Acceleron Pharma, Inc., Aerami Therapeutics, Bayer HealthCare Pharmaceuticals Inc., Janssen Biotech, Inc., Keros Therapeutics, Merck & Co., Inc., Pfizer Inc. and United Therapeutics Corp.; and employment for Pathos AI, Inc. (spouse/partner). M.M. Hoeper reports consultancy for Acceleron Pharma, Inc., Actelion Pharmaceuticals, Aerovate, AOP Orphan Pharmaceuticals, Bayer HealthCare, Ferrer Internacional SA, Gossamer Bio, Janssen Global Services, LLC, Keros and Merck & Co., Inc. M. Humbert reports grants/research support from Gossamer Bio and Merck & Co., Inc.; consultancy for 35 Pharma, Aerovate Therapeutics, Inc., AOP Orphan Pharmaceuticals, Bayer, Chiesi Farmaceutici SpA, Ferrer Internacional SA, Gossamer Bio, Janssen Pharmaceuticals, Keros Therapeutics, Liquidia Corp., Merck & Co., Inc., Novartis, Respira Therapeutics, Roivant Sciences Ltd and United Therapeutics Corp; honoraria from Janssen Pharmaceuticals and Merck & Co., Inc.; and data safety monitoring or advisory board work for 35 Pharma, Aerovate Therapeutics, Inc., Janssen Pharmaceuticals, Keros Therapeutics, Merck & Co., Inc., Novartis and United Therapeutics Corp. V.V. McLaughlin reports grants/research support from Aerovate Therapeutics, Inc., Altavant Sciences, Gossamer Bio, Janssen Biotech, Inc., Keros Therapeutics, Merck & Co., Inc. and Sonivie; consultancy for Aerami Therapeutics, Aerovate Therapeutics, Inc., Altavant Sciences, Bayer HealthCare, Caremark, Gossamer Bio, Janssen Biotech, Inc., Keros Therapeutics, Merck & Co., Inc., Roivant Sciences Ltd and United Therapeutics Corp.; and is fiduciary officer for Clene Inc. A.B. Waxman reports grants/research support from AI Therapeutics; consultancy for Aria CV, Gossamer Bio, Merck & Co. and United Therapeutics Corp.; and data and safety monitoring board work for Insmed Inc. S. Manimaran is a current employee of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., and may hold stock and/or stock options in Merck & Co., Inc. E. Mikhailova is a current employee of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., and may hold stock and/or stock options in Merck & Co., Inc. M. Reddy is a current employee of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., and may hold stock and/or stock options in Merck & Co., Inc. A. Lau is a current employee of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., and may hold stock and/or stock options in Merck & Co., Inc. J. de Oliveira Pena is a former employee of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., and may hold stock and/or stock options in Merck & Co., Inc. R. Souza reports consultancy for Acceleron Pharma, Inc., Bayer HealthCare Pharmaceuticals Inc. and Janssen Biotech, Inc.
Figures
Comment in
-
Soteria: deliverance from harm?Eur Respir J. 2025 Jul 24;66(1):2500697. doi: 10.1183/13993003.00697-2025. Print 2025 Jul. Eur Respir J. 2025. PMID: 40707163 No abstract available.
-
Telangiectasia, bleeding, and risk stratification: lessons from the SOTERIA study.Eur Respir J. 2025 Oct 16;66(4):2501685. doi: 10.1183/13993003.01685-2025. Print 2025 Oct. Eur Respir J. 2025. PMID: 41101934 No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
