Adaptive decision support for addiction treatment to implement initiation of buprenorphine for opioid use disorder in the emergency department: protocol for the ADAPT Multiphase Optimization Strategy trial
- PMID: 39979056
- PMCID: PMC11842997
- DOI: 10.1136/bmjopen-2024-098072
Adaptive decision support for addiction treatment to implement initiation of buprenorphine for opioid use disorder in the emergency department: protocol for the ADAPT Multiphase Optimization Strategy trial
Abstract
Introduction: Despite the current opioid crisis resulting in tens of thousands of deaths every year, buprenorphine, a medication that can reduce opioid-related mortality, withdrawal, drug use and craving, is still underprescribed in the emergency department (ED) for treatment of opioid use disorder (OUD). The EMergency department-initiated BuprenorphinE for opioid use Disorder (EMBED) trial introduced a clinical decision support (CDS) tool that improved the proportion of ED physicians prescribing buprenorphine but did not affect patient-level rates of buprenorphine initiation. The present trial aims to build on these findings by optimising CDS use through iterative improvements, refined interventions and clinician feedback to enhance OUD treatment initiation in EDs.
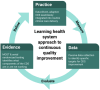
Methods and analysis: The Adaptive Decision support for Addiction Treatment (ADAPT) trial employs the Multiphase Optimization Strategy (MOST) framework to refine a multicomponent CDS tool designed to facilitate buprenorphine initiation for OUD in ED settings. Using a pragmatic, learning health system approach in three phases, the trial applies plan-do-study-act cycles for continuous CDS refinement. The CDS will be updated in the preparation phase to reflect new evidence. The optimisation phase will include a 2×2×2 factorial trial, testing the impact of various intervention components, followed by rapid, serial randomised usability testing to reduce user errors and enhance CDS workflow efficiency. In the evaluation phase, the optimised CDS package will be tested in a randomised trial to assess its effectiveness in increasing ED initiation of buprenorphine compared with the original EMBED CDS.
Ethics and dissemination: The protocol has received approval from our institution's institutional review board (protocol #2000038624) with a waiver of informed consent for collecting non-identifiable information only. Given the minimal risk involved in implementing established best practices, an independent study monitor will oversee the study instead of a Data Safety Monitoring Board. Findings will be submitted to ClinicalTrials.gov, published in open-access, peer-reviewed journals, presented at national conferences and shared with clinicians at participating sites through email notification.
Trial registration number: NCT06799117.
Keywords: Electronic Health Records; Health informatics; Randomized Controlled Trial.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: DRL is supported by the Department of Veterans Affairs, Veterans Health Administration, Office of Academic Affiliations, Office of Research and Development and has access to facilities at the VA Connecticut Healthcare System, West Haven, CT (CIN-13-407). All other authors have no competing interests to declare.
Figures

Similar articles
-
User-centred clinical decision support to implement emergency department-initiated buprenorphine for opioid use disorder: protocol for the pragmatic group randomised EMBED trial.BMJ Open. 2019 May 30;9(5):e028488. doi: 10.1136/bmjopen-2018-028488. BMJ Open. 2019. PMID: 31152039 Free PMC article.
-
Clinical decision support as an implementation strategy to expand identification and administration of treatment of opioid use disorder in the emergency department.J Subst Use Addict Treat. 2025 May;172:209653. doi: 10.1016/j.josat.2025.209653. Epub 2025 Feb 22. J Subst Use Addict Treat. 2025. PMID: 39993715
-
User centered clinical decision support to implement initiation of buprenorphine for opioid use disorder in the emergency department: EMBED pragmatic cluster randomized controlled trial.BMJ. 2022 Jun 27;377:e069271. doi: 10.1136/bmj-2021-069271. BMJ. 2022. PMID: 35760423 Free PMC article. Clinical Trial.
-
A practical review of buprenorphine utilization for the emergency physician in the era of decreased prescribing restrictions.Am J Emerg Med. 2021 Oct;48:316-322. doi: 10.1016/j.ajem.2021.06.065. Epub 2021 Jul 5. Am J Emerg Med. 2021. PMID: 34274576 Review.
-
Approach to buprenorphine use for opioid withdrawal treatment in the emergency setting.Am J Emerg Med. 2019 Jan;37(1):143-150. doi: 10.1016/j.ajem.2018.10.013. Epub 2018 Oct 11. Am J Emerg Med. 2019. PMID: 30355476 Review.
References
-
- Prescription opioid trends in the United States. [25-Jan-2023]. https://www.iqvia.com/en/insights/the-iqvia-institute/reports/prescripti... Available. Accessed.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
