Ethanol inhibits the growth and metastasis of hepatocellular carcinoma by inducing immunogenic cell death
- PMID: 39979070
- PMCID: PMC11843001
- DOI: 10.1136/jitc-2024-010472
Ethanol inhibits the growth and metastasis of hepatocellular carcinoma by inducing immunogenic cell death
Abstract
Background: Immunogenic cell death (ICD) can elicit an adaptive immune response with significant antitumor effects. Percutaneous ethanol injection therapy has been applied as tumor ablation for small hepatocellular carcinoma (HCC). However, it was not clear whether ethanol can elicit ICD. The aim of this study is to investigate the role of ethanol as an ICD inducer.
Methods: HCC cell lines were treated with low-concentration ethanol and ICD markers, such as calreticulin, high-mobility group box 1, and ATP were assayed. The mouse vaccination-rechallenge assay was used to further confirm ethanol as an ICD inducer. Western blot and real-time PCR were used to investigate ICD-related endoplasmic reticulum (ER) stress and signaling pathways. The genes with differential expression levels between primary and distant tumors were analyzed by nCounter gene expression. Intratumoral injection of ethanol was done to investigate the abscopal effect.
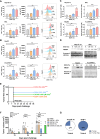
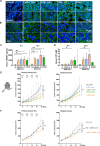
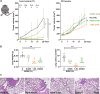
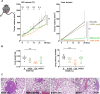
Results: The low-concentration ethanol could induce ICD in HCC cell lines through unfolded protein responses initiated by ER stress and multiple cell-death pathways. Intratumoral injections of low-concentration ethanol had significantly direct and abscopal antitumor effects in mouse models of both subcutaneous and orthotopic HCC. nCounter gene expression analysis in primary and distant tumors revealed the activation of various immune-response pathways, notably those mediated by CD8 T cells and the interferon pathway. Vaccinating mice with low-concentration ethanol-treated HCC cells successfully inhibited metastasis in both intravenous and intrasplenic metastasis models.
Conclusions: Our results suggest that low-concentration ethanol could serve as an inducer of ICD. Low-concentration ethanol could potentially improve therapeutic antitumor immunity by inducing substantial ICD.
Keywords: Abscopal; Hepatocellular Carcinoma; Immunotherapy.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: No, there are no competing interests.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
