Zika virus NS1 drives tunneling nanotube formation for mitochondrial transfer and stealth transmission in trophoblasts
- PMID: 39979240
- PMCID: PMC11842757
- DOI: 10.1038/s41467-025-56927-2
Zika virus NS1 drives tunneling nanotube formation for mitochondrial transfer and stealth transmission in trophoblasts
Abstract
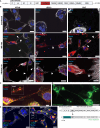
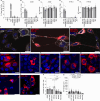
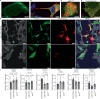
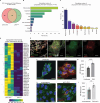
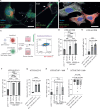
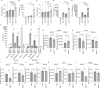
Zika virus (ZIKV) is unique among orthoflaviviruses in its vertical transmission capacity in humans, yet the underlying mechanisms remain incompletely understood. Here, we show that ZIKV induces tunneling nanotubes (TNTs) in placental trophoblasts which facilitate transfer of viral particles, proteins, mitochondria, and RNA to neighboring uninfected cells. TNT formation is driven exclusively via ZIKV non-structural protein 1 (NS1). Specifically, the N-terminal 1-50 amino acids of membrane-bound ZIKV NS1 are necessary for triggering TNT formation in host cells. Trophoblasts infected with TNT-deficient ZIKVΔTNT mutant virus elicited a robust antiviral IFN-λ 1/2/3 response relative to WT ZIKV, suggesting TNT-mediated trafficking allows ZIKV cell-to-cell transmission camouflaged from host defenses. Using affinity purification-mass spectrometry of cells expressing wild-type NS1 or non-TNT forming NS1, we found mitochondrial proteins are dominant NS1-interacting partners. We demonstrate that ZIKV infection or NS1 expression induces elevated mitochondria levels in trophoblasts and that mitochondria are siphoned via TNTs from healthy to ZIKV-infected cells. Together our findings identify a stealth mechanism that ZIKV employs for intercellular spread among placental trophoblasts, evasion of antiviral interferon response, and the hijacking of mitochondria to augment its propagation and survival and offers a basis for novel therapeutic developments targeting these interactions to limit ZIKV dissemination.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: I.U.M. serves on the scientific advisory board of Seed Health. The remaining authors declare no competing interests.
Figures


Update of
-
Zika Virus NS1 Drives Tunneling Nanotube Formation for Mitochondrial Transfer, Enhanced Survival, Interferon Evasion, and Stealth Transmission in Trophoblasts.Res Sq [Preprint]. 2023 Dec 6:rs.3.rs-3674059. doi: 10.21203/rs.3.rs-3674059/v1. Res Sq. 2023. Update in: Nat Commun. 2025 Feb 20;16(1):1803. doi: 10.1038/s41467-025-56927-2. PMID: 38106210 Free PMC article. Updated. Preprint.
References
-
- Duffy, M. R. et al. Zika virus outbreak on Yap Island, Federated States of Micronesia. N. Engl. J. Med.360, 2536–2543 (2009). - PubMed
MeSH terms
Substances
Grants and funding
- R01AI176505/Division of Intramural Research, National Institute of Allergy and Infectious Diseases (Division of Intramural Research of the NIAID)
- R01 AI176505/AI/NIAID NIH HHS/United States
- P30 CA125123/CA/NCI NIH HHS/United States
- S10 RR024574/RR/NCRR NIH HHS/United States
- R01 HD091218/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials

