Olaparib combined to metronomic cyclophosphamide and metformin in women with recurrent advanced/metastatic endometrial cancer: the ENDOLA phase I/II trial
- PMID: 39979249
- PMCID: PMC11842746
- DOI: 10.1038/s41467-025-56914-7
Olaparib combined to metronomic cyclophosphamide and metformin in women with recurrent advanced/metastatic endometrial cancer: the ENDOLA phase I/II trial
Abstract
Endometrial cancers are characterized by frequent alterations in the PI3K-AKT-mTor, IGF1 and DNA repair signaling pathways. Concomitant inhibition of these pathways was warranted. ENDOLA phase I/II trial (NCT02755844) was designed to assess the safety/efficacy of the triplet combination of the PARP inhibitor olaparib, metronomic cyclophosphamide (50 mg daily), and PI3K-AKT-mTor inhibitor metformin (1500 mg daily) in women with recurrent endometrial carcinomas. Olaparib dose-escalation (100-300 mg twice-a-day (bid)) was used to determine the recommended-phase II-trial-dose (RP2D, primary endpoint), followed by an expansion cohort to determine the non-progression rate at 10 weeks (NPR-10w, secondary endpoint). 31 patients were treated. Olaparib RP2D was defined as 300 mg bid. The tolerability was acceptable, and grade 3-4 adverse events (51% patients) were mainly hematological. The NPR-10w was 61.5%, and the median progression-free survival (mPFS) was 5.2 months. In a post-hoc analysis, when explored by molecular subtypes/alterations, longer PFS were observed in patients with tumors characterized by a non-specific-molecular-profile (NSMP, n = 4; mPFS, 9.1 months), and by both TP53 altered & high number of large genomic alterations (LGA ≥ 8)(n = 10, mPFS, 8.6 months)). The analyses about kinetics of circulating biomarkers and pharmacodynamic effects are not reported here. In total, the benefit/toxicity ratio of the all-oral olaparib/cyclophosphamide/metformin regimen was favorable in heavily pretreated patients with recurrent endometrial cancer.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: Florence Joly declares links of interest with AstraZeneca for expertise and the development of UTOLA trial. Gilles Freyer declares links of interest with AstraZeneca for expertise and consulting. Manuel Rodrigues reports personal fees for serving as an advisor for AstraZeneca; travel support from AstraZeneca; funds to his institution to support research from Merck Sharp & Dohme, Janssen-Cilag, Daiichi-Sankyo. Celine Callens is a co-inventor of the ShallowHRDv2 method (International Patent Application number PCT/EP2024/061709, filed on April 28th, 2024, published on October 31st, 2024 under the International publication number WO2024/223927 and entitled « METHODS FOR DIAGNOSING A HOMOLOGOUS RECOMBINATION DEFICIENCY IN HUMAN TUMORS »). Benoit You has links of interest with Astra-Zeneca for consulting, expertise and travel expenses Philippe Follana has links of interest with Astra-Zeneca for lectures, presentations, manuscript writing or educational events. Alexandra Leary has links of interest with Astra-Zeneca for travel to congress, fees for participation to boards, and funding for trials given to its institution. The other authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript, apart from those disclosed. No writing assistance was used in the production of this manuscript.
Figures

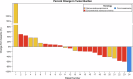

References
-
- Endometrial Cancer | Uterine Cancer | American Cancer Society n.d. https://www.cancer.org/cancer/types/endometrial-cancer.html (accessed 11 June 2024).
-
- Mirza, M. R. et al. Dostarlimab for primary advanced or recurrent endometrial cancer. N. Engl. J. Med.388, 2145–2158 (2023). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

