Optineurin-facilitated axonal mitochondria delivery promotes neuroprotection and axon regeneration
- PMID: 39979261
- PMCID: PMC11842812
- DOI: 10.1038/s41467-025-57135-8
Optineurin-facilitated axonal mitochondria delivery promotes neuroprotection and axon regeneration
Abstract
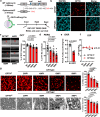
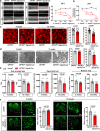
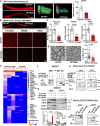
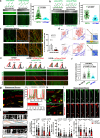
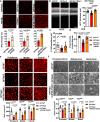
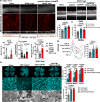
Optineurin (OPTN) mutations are linked to amyotrophic lateral sclerosis (ALS) and normal tension glaucoma (NTG), but a relevant animal model is lacking, and the molecular mechanisms underlying neurodegeneration are unknown. We find that OPTN C-terminus truncation (OPTN∆C) causes late-onset neurodegeneration of retinal ganglion cells (RGCs), optic nerve (ON), and spinal cord motor neurons, preceded by a decrease of axonal mitochondria in mice. We discover that OPTN directly interacts with both microtubules and the mitochondrial transport complex TRAK1/KIF5B, stabilizing them for proper anterograde axonal mitochondrial transport, in a C-terminus dependent manner. Furthermore, overexpressing OPTN/TRAK1/KIF5B prevents not only OPTN truncation-induced, but also ocular hypertension-induced neurodegeneration, and promotes robust ON regeneration. Therefore, in addition to generating animal models for NTG and ALS, our results establish OPTN as a facilitator of the microtubule-dependent mitochondrial transport necessary for adequate axonal mitochondria delivery, and its loss as the likely molecular mechanism of neurodegeneration.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: A PCT patent application entitled ‘Neuroprotection and Axon Regeneration Therapies for CNS Axonopathies By Modulating Mitochondria Trafficking through OPTN, TRAK1, and KIF5B’ has been filed by Stanford Office of Technology Licensing for international filing (International Application No. PCT/US2024/040324) for the AAV-mediated OPTN, TRAK1, and KIF5B overexpression identified in this manuscript. Yang Hu, Dong Liu, Haoliang Huang and Pingting Liu are coinventors, and Stanford University as the applicant of this patent. The other authors declare no competing interests.
Figures

Update of
-
Optineurin-facilitated axonal mitochondria delivery promotes neuroprotection and axon regeneration.bioRxiv [Preprint]. 2024 Apr 3:2024.04.02.587832. doi: 10.1101/2024.04.02.587832. bioRxiv. 2024. Update in: Nat Commun. 2025 Feb 20;16(1):1789. doi: 10.1038/s41467-025-57135-8. PMID: 38617277 Free PMC article. Updated. Preprint.
References
-
- Coleman, M. P. & Perry, V. H. Axon pathology in neurological disease: a neglected therapeutic target. Trends Neurosci.25, 532–537 (2002). - PubMed
-
- Raff, M. C., Whitmore, A. V. & Finn, J. T. Axonal self-destruction and neurodegeneration. Science296, 868–871 (2002). - PubMed
-
- Fischer, L. R. et al. Amyotrophic lateral sclerosis is a distal axonopathy: evidence in mice and man. Exp. Neurol.185, 232–240 (2004). - PubMed
-
- Nickells, R. W., Howell, G. R., Soto, I. & John, S. W. Under pressure: cellular and molecular responses during glaucoma, a common neurodegeneration with axonopathy. Annu Rev. Neurosci.35, 153–179 (2012). - PubMed
-
- Anderson, D. R., Drance, S. M., Schulzer, M. & Collaborative Normal-Tension Glaucoma Study G. Natural history of normal-tension glaucoma. Ophthalmology108, 247–253 (2001). - PubMed
MeSH terms
Substances
Grants and funding
- R01 EY034353/EY/NEI NIH HHS/United States
- P30 EY026877/EY/NEI NIH HHS/United States
- 1F32EY029567/U.S. Department of Health & Human Services | NIH | National Eye Institute (NEI)
- EY026877/U.S. Department of Health & Human Services | NIH | National Eye Institute (NEI)
- EY034353/U.S. Department of Health & Human Services | NIH | National Eye Institute (NEI)
- S10 OD025091/OD/NIH HHS/United States
- R01 EY032518/EY/NEI NIH HHS/United States
- EY032518/U.S. Department of Health & Human Services | NIH | National Eye Institute (NEI)
- R01 EY023295/EY/NEI NIH HHS/United States
- EY023295/U.S. Department of Health & Human Services | NIH | National Eye Institute (NEI)
- F32 EY029567/EY/NEI NIH HHS/United States
- R01 EY032159/EY/NEI NIH HHS/United States
- R01 EY024932/EY/NEI NIH HHS/United States
- EY024932/U.S. Department of Health & Human Services | NIH | National Eye Institute (NEI)
- R01 EY025295/EY/NEI NIH HHS/United States
- S10 OD030452/OD/NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

