Fc-engineered large molecules targeting blood-brain barrier transferrin receptor and CD98hc have distinct central nervous system and peripheral biodistribution
- PMID: 39979268
- PMCID: PMC11842567
- DOI: 10.1038/s41467-025-57108-x
Fc-engineered large molecules targeting blood-brain barrier transferrin receptor and CD98hc have distinct central nervous system and peripheral biodistribution
Abstract
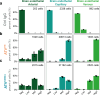
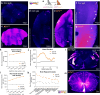
Blood brain barrier-crossing molecules targeting transferrin receptor (TfR) and CD98 heavy chain (CD98hc) are widely reported to promote enhanced brain delivery of therapeutics. Here, we provide a comprehensive and unbiased biodistribution characterization of TfR and CD98hc antibody transport vehicles (ATVTfR and ATVCD98hc) compared to control IgG. Mouse whole-body tissue clearing reveals distinct organ localization for each molecule. In the brain, ATVTfR and ATVCD98hc achieve enhanced exposure and parenchymal distribution even when brain exposures are matched between ATV and control IgG in bulk tissue. Using a combination of cell sorting and single-cell RNAseq, we reveal that control IgG is nearly absent from parenchymal cells and is distributed primarily to brain perivascular and leptomeningeal cells. In contrast, ATVTfR and ATVCD98hc exhibit broad and unique parenchymal cell-type distribution. Finally, we profile in detail brain region-specific biodistribution of ATVTfR in cynomolgus monkey brain and spinal cord. Taken together, this in-depth multiscale characterization will guide platform selection for therapeutic targets of interest.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: N.K., M.E.P., C.B.D., D.J., D.T., C.H., G.L.D., L.S., M.J.S., D.C., R.C., J.C., A.C., Y.R.C., J.C.D., J.D., H.K., A.L., E.L., A.M., E.R., T.S., M.T., K.X., Y.Z., J.L., R.G.T., M.E.K.C., and Y.J.Y.Z. are currently or were previously paid employees of Denali Therapeutics Inc. M.I.T., M.N., and D.K. are paid employees of Deep Piction and A.E. is the chief executive officer of Deep Piction.
Figures




References
-
- Banks, W. A. From blood-brain barrier to blood-brain interface: new opportunities for CNS drug delivery. Nat. Rev. Drug Discov.15, 275–292 (2016). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

