Invasion and metastasis in cancer: molecular insights and therapeutic targets
- PMID: 39979279
- PMCID: PMC11842613
- DOI: 10.1038/s41392-025-02148-4
Invasion and metastasis in cancer: molecular insights and therapeutic targets
Abstract
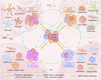
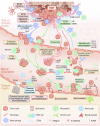
The progression of malignant tumors leads to the development of secondary tumors in various organs, including bones, the brain, liver, and lungs. This metastatic process severely impacts the prognosis of patients, significantly affecting their quality of life and survival rates. Research efforts have consistently focused on the intricate mechanisms underlying this process and the corresponding clinical management strategies. Consequently, a comprehensive understanding of the biological foundations of tumor metastasis, identification of pivotal signaling pathways, and systematic evaluation of existing and emerging therapeutic strategies are paramount to enhancing the overall diagnostic and treatment capabilities for metastatic tumors. However, current research is primarily focused on metastasis within specific cancer types, leaving significant gaps in our understanding of the complex metastatic cascade, organ-specific tropism mechanisms, and the development of targeted treatments. In this study, we examine the sequential processes of tumor metastasis, elucidate the underlying mechanisms driving organ-tropic metastasis, and systematically analyze therapeutic strategies for metastatic tumors, including those tailored to specific organ involvement. Subsequently, we synthesize the most recent advances in emerging therapeutic technologies for tumor metastasis and analyze the challenges and opportunities encountered in clinical research pertaining to bone metastasis. Our objective is to offer insights that can inform future research and clinical practice in this crucial field.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

