Bacteria invade the brain following intracortical microelectrode implantation, inducing gut-brain axis disruption and contributing to reduced microelectrode performance
- PMID: 39979293
- PMCID: PMC11842729
- DOI: 10.1038/s41467-025-56979-4
Bacteria invade the brain following intracortical microelectrode implantation, inducing gut-brain axis disruption and contributing to reduced microelectrode performance
Abstract
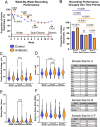
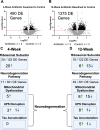
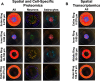
Brain-machine interface performance can be affected by neuroinflammatory responses due to blood-brain barrier (BBB) damage following intracortical microelectrode implantation. Recent findings suggest that certain gut bacterial constituents might enter the brain through damaged BBB. Therefore, we hypothesized that damage to the BBB caused by microelectrode implantation could facilitate microbiome entry into the brain. In our study, we found bacterial sequences, including gut-related ones, in the brains of mice with implanted microelectrodes. These sequences changed over time. Mice treated with antibiotics showed a reduced presence of these bacteria and had a different inflammatory response, which temporarily improved microelectrode recording performance. However, long-term antibiotic use worsened performance and disrupted neurodegenerative pathways. Many bacterial sequences found were not present in the gut or in unimplanted brains. Together, the current study established a paradigm-shifting mechanism that may contribute to chronic intracortical microelectrode recording performance and affect overall brain health following intracortical microelectrode implantation.
© 2025. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
Competing interests: The contents do not represent the views of the U.S. Department of Veterans Affairs, the National Institutes of Health, or the United States Government. The authors declare no conflict of interest.
Figures



Update of
-
Bacteria Invade the Brain Following Sterile Intracortical Microelectrode Implantation.Res Sq [Preprint]. 2024 Mar 7:rs.3.rs-3980065. doi: 10.21203/rs.3.rs-3980065/v1. Res Sq. 2024. Update in: Nat Commun. 2025 Feb 20;16(1):1829. doi: 10.1038/s41467-025-56979-4. PMID: 38496527 Free PMC article. Updated. Preprint.
Similar articles
-
Bacteria Invade the Brain Following Sterile Intracortical Microelectrode Implantation.Res Sq [Preprint]. 2024 Mar 7:rs.3.rs-3980065. doi: 10.21203/rs.3.rs-3980065/v1. Res Sq. 2024. Update in: Nat Commun. 2025 Feb 20;16(1):1829. doi: 10.1038/s41467-025-56979-4. PMID: 38496527 Free PMC article. Updated. Preprint.
-
The effect of resveratrol on neurodegeneration and blood brain barrier stability surrounding intracortical microelectrodes.Biomaterials. 2013 Sep;34(29):7001-15. doi: 10.1016/j.biomaterials.2013.05.035. Epub 2013 Jun 21. Biomaterials. 2013. PMID: 23791503
-
Differential expression of genes involved in the chronic response to intracortical microelectrodes.Acta Biomater. 2023 Oct 1;169:348-362. doi: 10.1016/j.actbio.2023.07.038. Epub 2023 Jul 26. Acta Biomater. 2023. PMID: 37507031 Free PMC article.
-
Understanding the Role of Innate Immunity in the Response to Intracortical Microelectrodes.Crit Rev Biomed Eng. 2018;46(4):341-367. doi: 10.1615/CritRevBiomedEng.2018027166. Crit Rev Biomed Eng. 2018. PMID: 30806249 Free PMC article. Review.
-
Potential for thermal damage to the blood-brain barrier during craniotomy: implications for intracortical recording microelectrodes.J Neural Eng. 2018 Jun;15(3):034001. doi: 10.1088/1741-2552/aa9f32. Epub 2017 Dec 5. J Neural Eng. 2018. PMID: 29205169 Free PMC article. Review.
References
-
- Bullard, A. J., Hutchison, B. C., Lee, J., Chestek, C. A. & Patil, P. G. Estimating risk for future intracranial, fully implanted, modular neuroprosthetic systems: a systematic review of hardware complications in clinical deep brain stimulation and experimental human intracortical arrays. Neuromodulation23, 411–426 (2020). - PubMed
-
- Biran, R., Martin, D. C. & Tresco, P. A. Neuronal cell loss accompanies the brain tissue response to chronically implanted silicon microelectrode arrays. Exp. Neurol.195, 115–126 (2005). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

