Site-specific activation of the proton pump inhibitor rabeprazole by tetrathiolate zinc centres
- PMID: 39979415
- PMCID: PMC11964933
- DOI: 10.1038/s41557-025-01745-8
Site-specific activation of the proton pump inhibitor rabeprazole by tetrathiolate zinc centres
Abstract
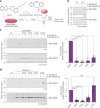
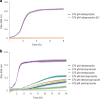
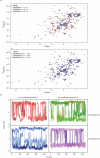
Proton pump inhibitors have become top-selling drugs worldwide. Serendipitously discovered as prodrugs that are activated by protonation in acidic environments, proton pump inhibitors inhibit stomach acid secretion by covalently modifying the gastric proton pump. Despite their widespread use, alternative activation mechanisms and potential target proteins in non-acidic environments remain poorly understood. Employing a chemoproteomic approach, we found that the proton pump inhibitor rabeprazole selectively forms covalent conjugates with zinc-binding proteins. Focusing on DENR, a protein with a C4 zinc cluster (that is, zinc coordinated by four cysteines), we show that rabeprazole is activated by the zinc ion and subsequently conjugated to zinc-coordinating cysteines. Our results suggest that drug binding, activation and conjugation take place rapidly within the zinc coordination sphere. Finally, we provide evidence that other proton pump inhibitors can be activated in the same way. We conclude that zinc acts as a Lewis acid, obviating the need for low pH, to promote the activation and conjugation of proton pump inhibitors in non-acidic environments.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures




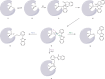





References
-
- World Health Organization Model List of Essential Medicines: 22nd List 62 (World Health Organization, 2021).
-
- Shin, J. M., Cho, Y. M. & Sachs, G. Chemistry of covalent inhibition of the gastric (H+, K+)-ATPase by proton pump inhibitors. J. Am. Chem. Soc.126, 7800–7811 (2004). - PubMed
-
- Sachs, G., Shin, J. M. & Howden, C. W. Review article: the clinical pharmacology of proton pump inhibitors. Aliment. Pharmacol. Ther.23, 2–8 (2006). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

