Secondary Hemophagocytic Lymphohistiocytosis in severe COVID-19 - a retrospective cohort study
- PMID: 39979496
- PMCID: PMC11842849
- DOI: 10.1038/s41598-025-90766-x
Secondary Hemophagocytic Lymphohistiocytosis in severe COVID-19 - a retrospective cohort study
Abstract
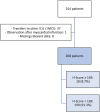
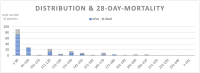
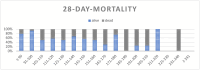
Hemophagocytic lymphohistiocytosis (HLH) is an excessive immune activation with cytokine storm und multi-organ dysfunction. It can occur secondarily, especially due to viral infections like COVID-19. Rapid treatment is crucial for favourable outcomes, but diagnosing HLH is challenging. The most common diagnostic instrument is the H-Score. However, the prognostic value of the H-score has not yet been assessed in detail in the spotlight of secondary HLH in severe COVID-19. COVID-19 patients treated between February 2020 and April 2021 at the intensive care unit (ICU) of the Department of Infectious Diseases and Tropical Medicine, Clinic Favoriten, Vienna, Austria, were included in this study. Data were assessed retrospectively by document review, and the follow-up period was at least 90 days. A total of 208 critically ill COVID-19 patients with an age of 61.8 ± 13.6 years were enrolled in this study. We found an average H-Score in the entire study collective of 94 ± 51 points, and 8.7% had a score ≥ 169 testing positive for HLH. A positive score was associated with increased mortality rates at 28 (66.7 vs. 26.3%, p < 0.001) and 90 days (72.2 vs. 27.9%, p < 0.001). In our cohort study, critically ill COVID-19 patients with an H-Score ≥ 169 during their ICU stay had increased mortality rates at 28 and 90 days. Thus, attention should be paid to individuals with rising or high scores. Therapeutic options and their impact on mortality for patients with COVID-19-associated secondary HLH should be evaluated in further studies.
Keywords: COVID-19; Critical care; H-Score; HLH; Haemophagocytic syndrome; Hemophagocytic lymphohistiocytosis; Intensive care medicine; Intensive care unit; SARS-CoV-2.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Ethical approval (N°20-079-VK) was provided by the Ethics Committee of the City of Vienna. Due to the retrospective nature of the study, the Ethics Committee of the City of Vienna waived the need of obtaining informed consent. The study complies with the Declaration of Helsinki and STROBE guidelines. Consent for publication: Informed consent (and therefore also consent for publication) was waived by the Ethics Committee. Competing interests: The authors declare no competing interests.
Figures

References
-
- 1. Ramos-Casals M, Brito-Zerón P, López-Guillermo A, Khamashta MA, Bosch X. Adult haemophagocytic syndrome. Lancet. 2014;383(9927):1503-16. - PubMed
-
- 2. Mou SS, Nakagawa TA, Riemer EC, McLean TW, Hines MH, Shetty AK. Hemophagocytic lymphohistiocytosis complicating influenza A infection. Pediatrics. 2006;118(1):e216-9. - PubMed
-
- 3. Harms PW, Schmidt LA, Smith LB, Newton DW, Pletneva MA, Walters LL, et al. Autopsy findings in eight patients with fatal H1N1 influenza. Am J Clin Pathol. 2010;134(1):27–35. - PubMed
-
- 4. Chen TL, Wong WW, Chiou TJ. Hemophagocytic syndrome: an unusual manifestation of acute human immunodeficiency virus infection. Int J Hematol. 2003;78(5):450-2. - PubMed
-
- 5. Fardet L, Blum L, Kerob D, Agbalika F, Galicier L, Dupuy A, et al. Human herpesvirus 8-associated hemophagocytic lymphohistiocytosis in human immunodeficiency virus-infected patients. Clin Infect Dis. 2003;37(2):285 − 91. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

