Intra and inter-organ communication through extracellular vesicles in obesity: functional role of obesesomes and steatosomes
- PMID: 39979938
- PMCID: PMC11844161
- DOI: 10.1186/s12967-024-06024-7
Intra and inter-organ communication through extracellular vesicles in obesity: functional role of obesesomes and steatosomes
Abstract
Background: Extracellular vesicles (EVs) represent a sophisticated mechanism of intercellular communication that is implicated in health and disease. Specifically, the role of EVs in metabolic regulation and their implications in metabolic pathologies, such as obesity and its comorbidities, remain unclear.
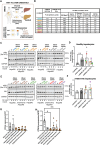
Methods: Extracellular vesicles (EVs) were isolated through serial ultracentrifugation from murine adipocytes treated with palmitate or oleic acid, whole visceral and subcutaneous adipose tissue (obesesomes) of bariatric surgery obese donors, and human hepatocytes under steatosis (steatosomes) for functional in vitro experiments. Functional effects on inflammation and glucose and lipid metabolism of target cells (human and murine macrophages and hepatocytes) were assessed using ELISA, RT-PCR, and immunodetection. Isolated EVs from human steatotic (steatosomes) and control hepatocytes (hepatosomes) were characterized for quantity, size, and tetraspanin profile by NTA and Single Particle Interferometric Reflectance Imaging Sensor (SP-IRIS), and their protein cargo analyzed by qualitative (DDA) and quantitative (DIA-SWATH) proteomics using LC-MS/MS. Proteins identified by proteomics were validated by capturing EVs on functionalized chips by SP-IRIS.
Results and conclusions: In this study, we investigated the role of EVs in the local communication between obese adipocytes and immune cells within adipose tissue, and the interaction of steatotic and healthy hepatocytes in the context of fatty liver disease progression. Furthermore, we analyzed obese adipose tissue-to-liver interactions through EV-obesesomes to elucidate their role in obesity-associated hepatic metabolic dysregulation. Our findings reveal that obesesomes promote inflammation and the secretion of pro-inflammatory cytokines upon interaction with macrophages, exerting a significant impact on reducing insulin resistance and altering lipid and glucose metabolism upon interaction with hepatocytes; in both cases, EVs from palmitate-loaded adipocytes and obesesomes from human visceral adipose depots demonstrated the most deleterious effect. Additionally, EVs secreted by steatotic hepatocytes (steatosomes) induced insulin resistance and altered lipid and glucose metabolism in healthy hepatocytes, suggesting their involvement in MASLD development. Proteomic analysis of steatosomes revealed that these vesicles contain liver disease-associated proteins, rendering them significant repositories of real-time biomarkers for the early stages and progression of MASLD.
Keywords: Extracellular vesicles; Inflammation; Liver steatosis; MASLD; Obesity.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All human samples and data were obtained after obtaining written informed consent, in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki). All procedures were approved by the Clinical Ethical Committee of Galicia (CEIC), Spain, under code number 2020/497. Competing interests: The authors declare that they have no affiliations with or involvement in any organization or entity with any financial interest in the subject matter or materials discussed in this manuscript.
Figures





References
-
- Francque SMA, Dirinck E. NAFLD prevalence and severity in overweight and obese populations. Lancet Gastroenterol Hepatol. 2023;8:2–3. - PubMed
-
- Tanaka M, Nozaki M, Fukuhara A, Segawa K, Aoki N, Matsuda M, et al. Visfatin is released from 3T3-L1 adipocytes via a non-classical pathway. Biochem Biophys Res Commun. 2007;359:194–201. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

