Genomic alterations are associated with response to aromatase inhibitor therapy for ER-positive postmenopausal ductal carcinoma in situ: (CALGB 40903, Alliance)
- PMID: 39980051
- PMCID: PMC11843815
- DOI: 10.1186/s13058-025-01963-5
Genomic alterations are associated with response to aromatase inhibitor therapy for ER-positive postmenopausal ductal carcinoma in situ: (CALGB 40903, Alliance)
Abstract
Purpose: CALGB 40903 (Alliance) was a phase II single arm multicenter trial conducted in postmenopausal patients diagnosed with estrogen-receptor (ER) positive breast ductal carcinoma in situ (DCIS) without invasion. Patients were treated with the aromatase inhibitor (AI) letrozole for 6 months prior to surgery with change in magnetic resonance imaging (MRI) enhancement volume compared to baseline as the primary endpoint. In the current study, we performed sequence analysis of pre- and post-treatment specimens to determine gene expression and DNA copy number parameters associated with treatment and response.
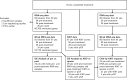
Experimental design: Paraffin sections from pretreatment biopsies and post-treatment surgical specimens were evaluated for presence of DCIS. Proliferation based on KI67 staining was quantified by a study pathologist. Macrodissection of the DCIS components from thin sections was the source of RNA and DNA. Whole-transcriptome RNA and shallow whole-genome DNA sequencing were performed. PAM50 analysis to assign intrinsic subtypes with associated probability of class membership was performed. Differential gene expression comparing responders versus non-responders and pre- versus post-treatment specimens was performed using a two-tiered approach based on candidate genes and a whole genome survey with appropriate multiple testing corrections.
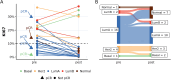
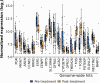
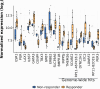
Results: Based on availability of specimens and presence of DCIS component, 29 patients (from the 70 who completed the treatment trial) were included in the final data set, including five who had a pathologic complete response (pCR). Response to treatment was qualified categorically based on a threshold of 10% KI67 in the post-treatment surgical specimen or pCR. Based on this criterion, six of the 29 DCIS were considered non-responders (> 10% KI67) and five subjects with pCR were assigned to the responder group. No standard clinical variables were associated with response. On the basis of gene expression analysis, 19 of the pre-treatment samples were classified as luminal A, all of which were classified as responders. PAM50 classification of the other ten pre-treatment samples included luminal B, HER2, basal, and normal-like, six of which were non-responders. PAM50 class membership shifted from baseline to post-treatment in eight cases, most often from luminal A to normal-like (five cases). Selected genes associated with estrogen receptor levels in invasive breast cancer were higher in AI responsive tumors. AI treatment resulted in reductions in estrogen and proliferation related genes.
Conclusions: Letrozole treatment produced an effective growth response, particularly in DCIS initially classified as luminal A. Study inclusion criteria of DCIS with at least 1% ER positive cells resulted in the inclusion of other subtypes that failed to respond. Treatment also induced both minor and major changes in intrinsic subtype based on PAM50 probabilities. Overall, these data indicate that response to AI treatment in ER( +) DCIS is variable and analogous to that observed in invasive breast cancers.
Translational relevance: Treatment for breast DCIS ranges from active surveillance to mastectomy, often combined with adjuvant endocrine therapy. The work presented here based on a unique neoadjuvant trial provides direct information on hormone therapy responsiveness of this disease and further couples the biology of invasive breast cancer to its non-obligate precursor.
Trial registration: ClinicalTrials.gov Identifier: NCT01439711.
Keywords: Breast cancer; Breast cancer surgery; DCIS; Ductal carcinoma in situ; Endocrine therapy; Letrozole; Neoadjuvant.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures
References
-
- Ellis MJ, Suman VJ, Hoog J, Lin L, Snider J, Prat A, Parker JS, Luo J, DeSchryver K, Allred DC, et al. Randomized phase II neoadjuvant comparison between letrozole, anastrozole, and exemestane for postmenopausal women with estrogen receptor-rich stage 2 to 3 breast cancer: clinical and biomarker outcomes and predictive value of the baseline PAM50-based intrinsic subtype–ACOSOG Z1031. J Clin Oncol. 2011;29(17):2342–9. - PMC - PubMed
-
- Ellis MJ, Tao Y, Luo J, A’Hern R, Evans DB, Bhatnagar AS, Chaudri Ross HA, von Kameke A, Miller WR, Smith I, et al. Outcome prediction for estrogen receptor-positive breast cancer based on postneoadjuvant endocrine therapy tumor characteristics. J Natl Cancer Inst. 2008;100(19):1380–8. - PMC - PubMed
-
- Ellis MJ, Suman VJ, Hoog J, Goncalves R, Sanati S, Creighton CJ, DeSchryver K, Crouch E, Brink A, Watson M, et al. Ki67 proliferation index as a tool for chemotherapy decisions during and after neoadjuvant aromatase inhibitor treatment of breast cancer: results from the American college of surgeons oncology group Z1031 trial (Alliance). J Clin Oncol. 2017;35(10):1061–9. - PMC - PubMed
-
- Hwang ES, Hyslop T, Hendrix LH, Duong S, Bedrosian I, Price E, Caudle A, Hieken T, Guenther J, Hudis CA, et al. Phase II single-arm study of preoperative letrozole for estrogen receptor-positive postmenopausal ductal carcinoma in situ: CALGB 40903 (Alliance). J Clin Oncol. 2020;38(12):1284–92. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

