A cluster randomized stepped wedge implementation trial of scale-up approaches to ending pregnancy-related and -associated morbidity and mortality disparities in 12 Michigan counties: rationale and study protocol
- PMID: 39980059
- PMCID: PMC11843809
- DOI: 10.1186/s43058-024-00677-7
A cluster randomized stepped wedge implementation trial of scale-up approaches to ending pregnancy-related and -associated morbidity and mortality disparities in 12 Michigan counties: rationale and study protocol
Abstract
Background: Hospital-focused maternal health safety and quality guidelines have been found to reduce pregnancy-related and -associated morbidity and mortality (PRAMM). Unfortunately, quality of obstetric care can improve without affecting disparities. This project is the first controlled implementation trial to test approaches to implementing safety guidelines that: (1) target PRAMM disparities; and (2) focus on community care (care provided outside hospitals in outpatient and other community settings, and coordination among care settings), where most deaths occur. It is also one of the first to test scale-up or sustainment implementation approaches to addressing maternal morbidity and mortality disparities.
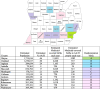
Methods: This project, one of three in the federally funded Multilevel Interventions for Raci.a.l Equity (MIRACLE) Maternal Health Research Center of Excellence, will develop and evaluate an implementation approach for scaling up bundled equity-focused maternal health safety guidelines in community care settings county-wide. The scale-up approach will be co-developed with partners, and then tested using a cluster randomized stepped-wedge trial of 12 Michigan counties with a total population of nearly 6 million. Randomization occurs at the county level; birthing people and providers are clustered within counties. PRAMM outcomes (individual level; primary) will be extracted from a pre-existing statewide linked dataset that includes Medicaid claims and vital records data. The sample will include all Medicaid insured individuals in the 12 counties observed during pregnancy, at birth, and up to 1 year postpartum during the project period (~ 151,920 births, including ~ 49,110 births to Black and/or Hispanic mothers). Implementation outcomes (provider level) will be collected using annual provider (n = 600) surveys and will include scale-up (penetration, reach, control for delivery, and intervention effectiveness at scale) and sustainment (maintenance of fidelity to core elements, health benefits, and capacity to deliver core elements over time) of bundles and cost-effectiveness of implementation approaches.
Discussion: This implementation trial will be the first to evaluate an implementation approach to scaling community health equity-focused maternal safety guidelines, addressing an understudied aspect of implementation science (i.e., scale-up). The study will also provide information about implementation cost-effectiveness needed to drive policy decisions.
Trial registration: The study was prospectively registered on Clinicaltrials.gov (NCT06541951) on August 6, 2024. The first participant has not yet been recruited. The url for the trial registration is: https://clinicaltrials.gov/study/NCT06541951?locStr=Flint,%20MI&country=United%20States&state=Michigan&city=Flint&rank=1 .
Keywords: Health inequities; Healthcare disparities; Implementation science; Maternal health; Maternal mortality.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This study was approved by the Michigan State University Biomedical Institutional Review Board (FWA00004556). Informed consent will be obtained from provider participants. Health data is obtained through a limited use administrative dataset. Consent for publication: NA. Competing interests: The authors declare that they have no competing interests.
Figures
Similar articles
-
Oxytocin for preventing postpartum haemorrhage (PPH) in non-facility birth settings.Cochrane Database Syst Rev. 2016 Apr 14;4(4):CD011491. doi: 10.1002/14651858.CD011491.pub2. Cochrane Database Syst Rev. 2016. PMID: 27078125 Free PMC article.
-
Sexual Harassment and Prevention Training.2024 Mar 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Mar 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 36508513 Free Books & Documents.
-
Interventions for preventing and reducing the use of physical restraints of older people in general hospital settings.Cochrane Database Syst Rev. 2022 Aug 25;8(8):CD012476. doi: 10.1002/14651858.CD012476.pub2. Cochrane Database Syst Rev. 2022. PMID: 36004796 Free PMC article.
-
Community-based intervention packages for reducing maternal and neonatal morbidity and mortality and improving neonatal outcomes.Cochrane Database Syst Rev. 2015 Mar 23;2015(3):CD007754. doi: 10.1002/14651858.CD007754.pub3. Cochrane Database Syst Rev. 2015. PMID: 25803792 Free PMC article.
-
Perceptions and experiences of the prevention, detection, and management of postpartum haemorrhage: a qualitative evidence synthesis.Cochrane Database Syst Rev. 2023 Nov 27;11(11):CD013795. doi: 10.1002/14651858.CD013795.pub2. Cochrane Database Syst Rev. 2023. PMID: 38009552 Free PMC article.
References
-
- Pregnancy Mortality Surveillance System. Centers for Disease Control and Prevention. 2023. https://www.cdc.gov/reproductivehealth/maternal-mortality/pregnancy-mort.... Accessed 1 May 2020.
-
- Four in 5 pregnancy-related deaths in the U.S. are preventable. CDC Newsroom. 2022. https://www.cdc.gov/media/releases/2022/p0919-pregnancy-related-deaths.h.... Accessed 7 Nov 2022.
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical