Lung-delivered IL-10 mitigates Lung inflammation induced by repeated endotoxin exposures in male mice
- PMID: 39980189
- PMCID: PMC11842461
- DOI: 10.14814/phy2.70253
Lung-delivered IL-10 mitigates Lung inflammation induced by repeated endotoxin exposures in male mice
Abstract
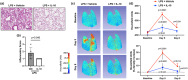
Therapies capable of resolving inflammatory lung disease resulting from high-consequence occupational/environmental hazards are lacking. This study seeks to determine the therapeutic potential of direct lung-delivered interleukin (IL)-10 following repeated lipopolysaccharide exposures. C57BL/6 mice were intratracheally instilled with LPS (10 μg) and treated with IL-10 (1 μg) or vehicle control for 3 days. Lung cell infiltrates were enumerated by flow cytometry. Lung sections were stained for myeloperoxidase (MPO), CCR2, vimentin, and post-translational protein citrullination (CIT) and malondialdehyde-acetaldehyde (MAA) modifications. Lung function testing and longitudinal in vivo micro-CT imaging were performed. Whole lungs were profiled using bulk RNA sequencing. IL-10 treatment reduced LPS-induced weight loss, pentraxin-2, and IL-6 serum levels. LPS-induced lung proinflammatory and wound repair mediators (i.e., TNF-α, IL-6, CXCL1, CCL2, MMP-8, MMP-9, TIMP-1, fibronectin) were decreased with IL-10. IL-10 reduced LPS-induced influx of lung neutrophils, CD8+ T cells, NK cells, recruited monocyte-macrophages, monocytes, and tissue expression of CCR2+ monocytes-macrophages, MPO+ neutrophils, vimentin, CIT, and MAA. IL-10 reduced LPS-induced airway hyperresponsiveness and improved lung compliance. Micro-CT imaging confirmed the reduction in LPS-induced lung density by IL-10. Lung-delivered IL-10 therapy administered after daily repeated endotoxin exposures strikingly reduces lung inflammatory and wound repair processes to decrease lung pathologic changes and mitigate airway dysfunction.
Keywords: endotoxin; environmental lung disease; inflammation; macrophages; occupational.
© 2025 The Author(s). Physiological Reports published by Wiley Periodicals LLC on behalf of The Physiological Society and the American Physiological Society.
Conflict of interest statement
JAP has received research reagents (anti‐IL‐33/ST2 blocking antibody reagent, no monies) from AstraZeneca. JAP is a site recruiter for clinical industry studies for asthma, sinus disease, and urticaria (GlaxoSmithKline, AstraZeneca, Regeneron Pharmaceuticals, CellDex Therapeutics). TRM has consulted for Horizon Therapeutics, Otaltech Therapeutics, Pfizer, and UCB and receives research support from Horizon.
Figures

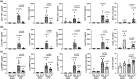




References
-
- Albright, M. , Guttenberg, M. A. , & Tighe, R. M. (2022). Ozone‐induced models of airway Hyperreactivity and epithelial injury. Methods in Molecular Biology, 2506, 67–81. - PubMed
-
- Benjamini, Y. , & Hochberg, Y. (1995). Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society: Series B: Methodological, 57(1), 289–300.
-
- Blazquez‐Prieto, J. , Lopez‐Alonso, I. , Huidobro, C. , & Albaiceta, G. M. (2018). The emerging role of neutrophils in repair after acute Lung injury. American Journal of Respiratory Cell and Molecular Biology, 59(3), 289–294. - PubMed
-
- Brandenberger, C. , & Muhlfeld, C. (2017). Mechanisms of lung aging. Cell and Tissue Research, 367(3), 469–480. - PubMed
MeSH terms
Substances
Grants and funding
- BX004660/U.S. Department of Veterans Affairs (VA)
- R01OH012045/HHS | CDC | National Institute for Occupational Safety and Health (NIOSH)
- R01 OH012045/OH/NIOSH CDC HHS/United States
- IK6 BX005962/BX/BLRD VA/United States
- BX003635/U.S. Department of Veterans Affairs (VA)
- F30 ES036063/ES/NIEHS NIH HHS/United States
- P30 CA036727/CA/NCI NIH HHS/United States
- PR200793/U.S. Department of Defense (DOD)
- 1F30ES036063-01/HHS | NIH | National Institute of Environmental Health Sciences (NIEHS)
- P20 GM103427/GM/NIGMS NIH HHS/United States
- 2U54GM115458/GF/NIH HHS/United States
- U54 GM115458/GM/NIGMS NIH HHS/United States
- I01 BX004660/BX/BLRD VA/United States
- BX005962/U.S. Department of Veterans Affairs (VA)
- I01 BX003635/BX/BLRD VA/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

