MET variants with activating N-lobe mutations identified in hereditary papillary renal cell carcinomas still require ligand stimulation
- PMID: 39980226
- PMCID: PMC12330938
- DOI: 10.1002/1878-0261.13806
MET variants with activating N-lobe mutations identified in hereditary papillary renal cell carcinomas still require ligand stimulation
Abstract
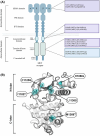
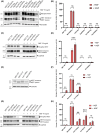
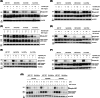
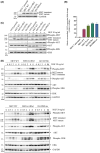
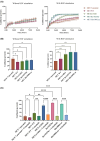
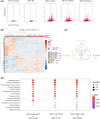
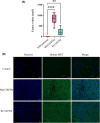
In hereditary papillary renal cell carcinoma (HPRCC), the hepatocyte growth factor receptor (MET) receptor tyrosine kinase (RTK) mutations recorded to date are located in the kinase domain and lead to constitutive MET activation. This contrasts with MET mutations identified in non-small-cell lung cancer (NSCLC), which lead to exon 14 skipping and deletion of a regulatory domain: In this latter case, the mutated receptor still requires ligand stimulation. Sequencing of MET in samples from 158 HPRCC and 2808 NSCLC patients revealed 10 uncharacterized mutations. Four of these, all found in HPRCC and leading to amino acid substitutions in the N-lobe of the MET kinase, proved able to induce cell transformation, which was further enhanced by hepatocyte growth factor (HGF) stimulation: His1086Leu, Ile1102Thr, Leu1130Ser, and Cis1125Gly. Similar to the variant resulting in MET exon 14 skipping, the two N-lobe MET variants His1086Leu and Ile1102Thr were found to require stimulation by HGF in order to strongly activate downstream signaling pathways and epithelial cell motility. The Ile1102Thr mutation also displayed transforming potential, promoting tumor growth in a xenograft model. In addition, the N-lobe-mutated MET variants were found to trigger a common HGF-stimulation-dependent transcriptional program, consistent with an observed increase in cell motility and invasion. Altogether, this functional characterization revealed that N-lobe variants still require ligand stimulation, in contrast to other RTK variants. This suggests that HGF expression in the tumor microenvironment is important for tumor growth. The sensitivity of these variants to MET inhibitors opens the way for use of targeted therapies for patients harboring the corresponding mutations.
Keywords: MET; cancer; hepatocyte growth factor; hereditary papillary renal cancer; receptor tyrosine kinase; somatic mutations.
© 2025 The Author(s). Molecular Oncology published by John Wiley & Sons Ltd on behalf of Federation of European Biochemical Societies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Update of
-
MET variants with activating N-lobe mutations identified in hereditary papillary renal cell carcinomas still require ligand stimulation.bioRxiv [Preprint]. 2025 Feb 25:2023.11.03.565283. doi: 10.1101/2023.11.03.565283. bioRxiv. 2025. Update in: Mol Oncol. 2025 Aug;19(8):2366-2387. doi: 10.1002/1878-0261.13806. PMID: 37965202 Free PMC article. Updated. Preprint.
References
-
- Manning G, Whyte DB, Martinez R, Hunter T, Sudarsanam S. The protein kinase complement of the human genome. Science. 2002;298(5600):1912–1934. - PubMed
-
- Robinson DR, Wu YM, Lin SF. The protein tyrosine kinase family of the human genome. Oncogene. 2000;19(49):5548–5557. - PubMed
-
- Hubbard SR. Structural analysis of receptor tyrosine kinases. Prog Biophys Mol Biol. 1999;71(3):343–358. - PubMed
-
- Bedard PL, Hyman DM, Davids MS, Siu LL. Small molecules, big impact: 20 years of targeted therapy in oncology. Lancet. 2020;395(10229):1078–1088. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

