Interim 2024/25 influenza vaccine effectiveness: eight European studies, September 2024 to January 2025
- PMID: 39980423
- PMCID: PMC11843620
- DOI: 10.2807/1560-7917.ES.2025.30.7.2500102
Interim 2024/25 influenza vaccine effectiveness: eight European studies, September 2024 to January 2025
Erratum in
-
Erratum for Euro Surveill. 2025;30(7).Euro Surveill. 2025 Mar;30(11):240320c. doi: 10.2807/1560-7917.ES.2025.30.11.240320c. Euro Surveill. 2025. PMID: 40116029 Free PMC article. No abstract available.
Abstract
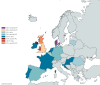
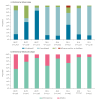
The 2024/25 influenza season in Europe is currently characterised by co-circulation of influenza A(H1N1)pdm09, A(H3N2) and B/Victoria viruses, with influenza A(H1N1)pdm09 predominating. Interim vaccine effectiveness (VE) estimates from eight European studies (17 countries) indicate an all-age influenza A VE of 32-53% in primary care and 33-56% in hospital settings, with some signals of lower VE by subtype and higher VE against influenza B (≥ 58% across settings). Where feasible, influenza vaccination should be encouraged and other prevention measures strengthened.
Keywords: Europe; influenza; multicentre study; test-negative design; vaccine effectiveness.
Conflict of interest statement
None of the other authors has declared any conflict of interest.
Figures

References
-
- World Health Organization (WHO). Recommended composition of influenza virus vaccines for use in the 2024-2025 northern hemisphere influenza season. Geneva: WHO; 2024. Available from: https://cdn.who.int/media/docs/default-source/influenza/who-influenza-re...
-
- Kissling E, Valenciano M, Falcao J, Larrauri A, Widgren K, Pitigoi D, et al. "I-MOVE" towards monitoring seasonal and pandemic influenza vaccine effectiveness: lessons learnt from a pilot multi-centric case-control study in Europe, 2008-9. Euro Surveill. 2009;14(44):19388. 10.2807/ese.14.44.19388-en - DOI - PubMed
-
- Kissling E, Pozo F, Martínez-Baz I, Buda S, Vilcu A-M, Domegan L, et al. Influenza vaccine effectiveness against influenza A subtypes in Europe: Results from the 2021-2022 I-MOVE primary care multicentre study. Influenza Other Respir Viruses. 2023;17(1):e13069. 10.1111/irv.13069 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
