Fission yeast cells deficient in siderophore biosynthesis require Str2 for ferrichrome-dependent growth
- PMID: 39980688
- PMCID: PMC11840675
- DOI: 10.3389/fmicb.2025.1527727
Fission yeast cells deficient in siderophore biosynthesis require Str2 for ferrichrome-dependent growth
Abstract
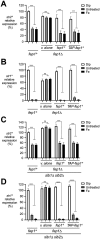
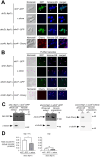
Ferrichrome (Fc) acquisition in Schizosaccharomyces pombe is mediated by the cell-surface siderophore-iron transporter Str1. Here, we report that Str2, a protein homologous to Str1, localizes to the vacuolar membrane. Like Str1, Str2 expression is transcriptionally regulated in response to changes in iron concentrations. Both the str2+ and str1+ genes are induced under low-iron conditions and are repressed by the iron-responsive GATA-type transcription factor Fep1 when iron is abundant. Under high-iron conditions, chromatin immunoprecipitation (ChIP) assays reveal that TAP-Fep1 occupies the str2+ and str1+ promoters. Isolated vacuoles from str2Δ fep1Δ cells expressing GFP-tagged Str2 exhibit iron accumulation in vacuoles upon exposure to exogenous holo-Fc. sib1Δ sib2Δ cells deficient in Fc biosynthesis and lacking the str2+ gene (str2Δ) are unable to grow in the presence of exogenous Fc as a sole source of iron. Further analysis identified that conserved amino acids Tyr539 and Tyr553 in the last predicted loop of Str2 are required for supporting Fc-dependent growth of a sib1Δ sib2Δ mutant strain. Collectively, these findings indicate that the vacuolar Str2 protein plays a role in the consumption of Fc as an iron source, while also revealing the involvement of the vacuole in iron release from exogenous Fc after its assimilation.
Keywords: ferrichrome; fission yeast; iron; iron-regulatory GATA-type transcription factor; siderophore transporter.
Copyright © 2025 Mbuya, Plante, Vahsen, Brault and Labbé.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
LinkOut - more resources
Full Text Sources
Miscellaneous

