Recent advances in systemic therapy for advanced biliary tract cancer: A systematic review and meta-analysis using reconstructed RCT survival data
- PMID: 39980751
- PMCID: PMC11840543
- DOI: 10.1016/j.jhepr.2024.101290
Recent advances in systemic therapy for advanced biliary tract cancer: A systematic review and meta-analysis using reconstructed RCT survival data
Abstract
Background & aims: Gemcitabine/cisplatin (GemCis) was the long-standing first-line treatment for advanced biliary tract cancers (BTCs). Following positive results from the TOPAZ-01 and KEYNOTE-966 trials, immune checkpoint inhibitors (ICIs) combined with chemotherapy are now the standard of care. We aim to compare the efficacy of first-line therapies for advanced BTCs.
Methods: Our systematic review included studies from five databases focusing on English-language articles published between January 2010 and June 2024. We included randomized clinical trials (RCTs) that featured GemCis in a treatment arm for treatment-naive adults with advanced BTCs. The primary endpoints were overall survival (OS) and progression-free survival. We conducted a one-stage meta-analysis using reconstructed survival data, Cox-based models, and restricted mean survival time (RMST).
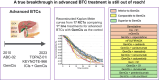
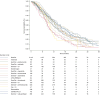
Results: After screening 8,797 studies, 17 RCTs were selected, involving a total of 4,584 patients. Of these, 2,140 (46.7%) received GemCis. The majority (68.9%) were diagnosed with intrahepatic or extrahepatic cholangiocarcinoma, and 80% had metastatic disease at the time of treatment. The pooled median OS in the GemCis group was 11.6 months (95% CI 11.3-12.2 months). GemCis plus pembrolizumab (hazard ratio [HR] 0.99, 95% CI 0.98-0.99; p <0.001), GemCis plus durvalumab (HR 0.98, 95% CI 0.97-0.99; p = 0.015), GemCis plus S-1 (HR 0.97 95% CI 0.95-0.99; p <0.001), and GemCis plus nab-paclitaxel (HR 0.98, 95% CI 0.98-0.99; p <0.001) demonstrated superior OS compared with GemCis alone. These combinations also showed increases in RMST by +1.1, +2.5, +2.8, and +2.1 months, respectively. In terms of progression-free survival, GemCis with ICIs (HR 0.91, 95% CI 0.78-0.94; p <0.001), GemCis plus S-1 (HR 0.98, 95% CI 0.96-0.99; p = 0.003), and GemCis plus nab-paclitaxel (HR 0.98, 95% CI 0.97-0.99; p <0.001) also demonstrated superiority, with corresponding RMST increases of +0.7, +1.9, and +2.5 months, respectively.
Conclusions: Despite incremental advancements, a breakthrough in advanced BTC treatment remains elusive. Further improvements in treatment efficacy may require biomarker identification to optimize combinational therapies for better patient selection.
Impact and implications: This study analyzed recent RCTs, including KEYNOTE-966, TOPAZ-1, NIFE, and SWOG 1815, involving 4,584 patients with advanced biliary tract cancer. A meta-analysis of 17 treatment arms, using reconstructed survival data, confirmed the modest survival benefit of GemCis plus ICIs, supporting its guideline adoption. The findings, however, highlight the need for biomarker identification and better patient selection.
Keywords: Biliary tract cancers; Cholangiocarcinoma; First-line therapy; Immunotherapy; Meta-analysis; Systematic review; Systemic therapy.
© 2024 The Authors.
Conflict of interest statement
AV had been directly paid honoraria and has been paid consulting or advisory roles for AstraZeneca, BeiGene, Boehringer Mannheim, BMS, BTG, EISAI, GSK, Incyte, Ipsen, MSD, Hoffmann-La Roche, Servier, Sirtex, and Taiho. GS discloses consultancy for AstraZeneca, Roche, Evidera, Novartis, HepaRegenix, and Integra; has received financial compensation for talks for Roche, AstraZeneca, Chiesi, and Integra; has received grants from Roche and AstraZeneca. GO has received grants from Roche and AstraZeneca; consulting fees from AstraZeneca, Servier, and Incyte; payment for lectures from Roche; and support for attending meetings from MSD and Roche. None of the other authors have any conflicts of interest to declare. Please refer to the accompanying ICMJE disclosure forms for further details.
Figures


References
-
- Forner A., Vidili G., Rengo M., et al. Clinical presentation, diagnosis and staging of cholangiocarcinoma. Liver Int. 2019;39(Suppl 1):98–107. - PubMed
-
- Valle J., Wasan H., Palmer D.H., et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273–1281. - PubMed
LinkOut - more resources
Full Text Sources

