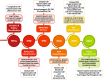
The 'Padua classification' of cardiomyopathies into three groups: hypertrophic/restrictive, dilated/hypokinetic, and scarring/arrhythmogenic
- PMID: 39980775
- PMCID: PMC11836707
- DOI: 10.1093/eurheartjsupp/suae108
The 'Padua classification' of cardiomyopathies into three groups: hypertrophic/restrictive, dilated/hypokinetic, and scarring/arrhythmogenic
Abstract
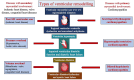
The newly proposed classification of cardiomyopathies, referred to as 'the Padua Classification', is based on both pathobiological basis (genetics, molecular biology, and pathology) and clinical features (morpho-functional and structural ventricular remodelling as evidenced by cardiac magnetic resonance). Cardiomyopathies are grouped into tree main categories and characterized by a designation combining both 'anatomical' and 'functional' features: hypertrophic/restrictive, dilated/hypokinetic, and scarring/arrhythmogenic; each cardiomyopathy group includes either genetic or non-genetic aetiologic variants. This novel approach aims to enhance the diagnostic accuracy and to support 'disease-specific' therapeutic strategies, with the objective to improve patient management and outcome.
Keywords: Cardiac magnetic resonance; Cardiomyopathy; Diagnosis.
© The Author(s) 2025. Published by Oxford University Press on behalf of the European Society of Cardiology.
Conflict of interest statement
Conflict of interest: None declared.
Figures


References
-
- Braunwald E. Cardiomyopathies: an overview. Circ Res 2017;121:711–721. - PubMed
-
- McKenna WJ, Maron BJ, Thiene G. Classification, epidemiology, and global burden of cardiomyopathies. Circ Res 2017;121:722–730. - PubMed
-
- Thiene G, Corrado D, Basso C. Revisiting definition and classification of cardiomyopathies in the era of molecular medicine. Eur Heart J 2008;29:144–146. - PubMed
LinkOut - more resources
Full Text Sources
