Pre-transplant T-cell clonal analysis identifies CD8+ donor reactive clones that contribute to kidney transplant rejection
- PMID: 39981250
- PMCID: PMC11840674
- DOI: 10.3389/fimmu.2025.1516772
Pre-transplant T-cell clonal analysis identifies CD8+ donor reactive clones that contribute to kidney transplant rejection
Abstract
Introduction: Responses to allogeneic human leukocyte antigen (HLA) molecules limit the survival of transplanted organs. The changes in T-cell alloreactivity that contribute to this process, however, are not fully understood. We defined a set of donor reactive T-cell clones (DRTC) with the goal to elucidate signatures of kidney allograft rejection.
Methods: DRTC were identified pretransplant using an anti-donor mixed lymphocyte reaction assay: CFSE-diluting CD4+ and CD8+ DRTC were flow-sorted, and the TCR sequences were identified using Adaptive Immunosequencing. DRTC were then tracked in post-transplant biopsies, blood, and urine samples in a cohort of kidney transplant recipients.
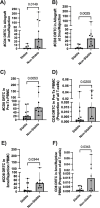
Results: In patients with an abnormal biopsy, the majority of CD8+ DRTC found within the allograft were detected in the circulating pre-transplant repertoire. Circulating CD8+ DRTC were more abundant pre- and post-transplant in patients that received non-lymphodepletional induction and developed an abnormal biopsy when compared to stable patients. Additionally, DRTC were detected as early as two weeks post-transplant in the urine of some patients, with some of these clones subsequently identified in follow-up kidney biopsy samples.
Discussion: The findings of our study add to our understanding of T-cell alloreactivity following kidney transplantation and provide evidence for the role of pre-defined alloreactive T-cells in the development of allograft rejection.
Keywords: T-cell mediated rejection; T-cell receptor sequencing; alloreactivity; kidney transplant rejection; mechanisms of rejection.
Copyright © 2025 Sanders, Banbury, Schumacher, He, Sambandam, Fields, Gallon, Mathew and Leventhal.
Conflict of interest statement
BB and ES are both salaried employees and own stock options of Adaptive Biotechnologies. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
-
- Tanriover B, Jaikaransingh V, MacConmara MP, Parekh JR, Levea SL, Ariyamuthu VK, et al. . Acute rejection rates and graft outcomes according to induction regimen among recipients of kidneys from deceased donors treated with tacrolimus and mycophenolate. Clin J Am Soc Nephrol. (2016) 11:1650–61. doi: 10.2215/CJN.13171215 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

