Comparison of air-liquid interface transwell and airway organoid models for human respiratory virus infection studies
- PMID: 39981254
- PMCID: PMC11839712
- DOI: 10.3389/fimmu.2025.1532144
Comparison of air-liquid interface transwell and airway organoid models for human respiratory virus infection studies
Abstract
Introduction: Complex in vitro respiratory models, including air-liquid interface (ALI) transwell cultures and airway organoids, have emerged as promising tools for studying human respiratory virus infections. These models address several limitations of conventional two-dimensional cell line and animal models. However, the lack of standardized protocols for the application of these models in infection studies limits the possibilities for comparing results across different research groups. Therefore, we applied a collaborative approach to harmonize several aspects of experimental methodology between different research laboratories, aiming to assess the comparability of different models of human airway epithelium in the context of respiratory viral infections.
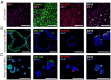
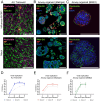
Methods: In this study, we compared three different models of human respiratory epithelium: a primary human bronchial epithelial cell-derived ALI transwell model, and two airway organoid models established from human airway- and lung-derived adult stem cells. We first assessed the presence of various differentiated cell types using immunofluorescence microscopy. Using a shared stock of influenza A virus, we then assessed viral growth kinetics, epithelial cytokine responses, and serum-mediated inhibition of infection.
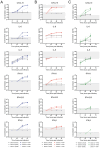
Results: The presence of club, goblet, and ciliated cells was confirmed in all models. We observed similar viral replication kinetics with a >4-log increase in virus titre across all models using a TCID50 assay. Following infection, a reproducible antiviral cytokine response, including a consistent increase in CXCL10, IL-6, IFN-λ1, IFN-λ2/3, and IFN-β, was detected across all models. Finally, neutralization was assessed by pre-incubation of virus with human serum. Reduced viral replication was observed across all models, resulting in a 3- to 6-log decrease in virus titres as quantified by TCID50.
Discussion: In conclusion, all three models produced consistent results regardless of the varying cell sources, culturing approaches, and infection methods. Our collaborative efforts to harmonize infection experiments and compare ALI transwell and airway organoid models described here aid in advancing our understanding and improving the standardization of these complex in vitro respiratory models for future studies.
Keywords: complex in vitro models; harmonization; influenza virus; mucosal models; respiratory tract.
Copyright © 2025 Ekanger, Dinesh Kumar, Koutstaal, Zhou, Beukema, Waldock, Jochems, Mulder, van Els, Engelhardt, Mantel, Buno, Brokstad, Engelsen, Cox, Melgert, Huckriede and van Kasteren.
Conflict of interest statement
NM is a Sanofi employee and may hold shares and/or stock options in the company. KPB is an employee of the GSK group of companies. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

