Metagenomic next-generation sequencing assists in the diagnosis of visceral leishmaniasis in non-endemic areas of China
- PMID: 39981377
- PMCID: PMC11839618
- DOI: 10.3389/fcimb.2025.1517046
Metagenomic next-generation sequencing assists in the diagnosis of visceral leishmaniasis in non-endemic areas of China
Abstract
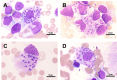
Introduction: Leishmaniasis, a protozoan disease caused by infection by Leishmania, is a critical issue in Asia, South America, East Africa, and North Africa. With 12 million cases globally, leishmaniasis is one of the most serious neglected tropical diseases worldwide. Direct identification of infected tissues is currently the primary method of diagnosis; however, the low sensitivity and inconvenience of microscopic examination in detecting amastigotes, parasitic manifestations of Leishmania, leads to the possibility of misdiagnosis, delayed diagnosis, and underdiagnosis.
Methods: With the development of metagenomic nextgeneration sequencing (mNGS) technology for pathogen identification, it is possible to detect specific nucleic acid sequences characteristic of Leishmania parasites, which opens new avenues for the more accurate diagnosis of leishmaniasis. In this study, we report two cases of leishmaniasis from Henan Province, China, in which Leishmania parasites were identified using mNGS technology, massively expediting diagnosis and treatment.
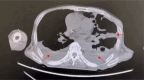
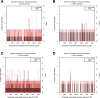
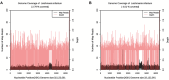
Results: Our report demonstrates that the mNGS method is applicable to peripheral blood samples (PB), which are far more readily available in clinical settings, in addition to bone marrow aspirate samples (BM), which are traditionally used for diagnosis of visceral leishmaniasis.
Conclusion: Our report validates the efficacy of mNGS technology as a rapid and accurate method of diagnosis for leishmaniasis.
Keywords: clinical diagnosis; endemic area; leishmaniasis; metagenomic next-generation sequencing assists; therapy.
Copyright © 2025 Zhao, He, Xiang, Ji, He and Wei.
Conflict of interest statement
Author GH and LX were employed by the company Nanjing Practice Medicine Diagnostics CO., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Leishmaniasis Diagnosis via Metagenomic Next-Generation Sequencing.Front Cell Infect Microbiol. 2020 Sep 23;10:528884. doi: 10.3389/fcimb.2020.528884. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 33072623 Free PMC article.
-
Metagenomic Analysis Identifying a Rare Leishmania Infection in an Adult With AIDS.Front Cell Infect Microbiol. 2021 Dec 15;11:764142. doi: 10.3389/fcimb.2021.764142. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 34976855 Free PMC article.
-
[Auxiliary diagnosis of visceral leishmaniasis with metagenomic next-generation sequencing: a case report].Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi. 2024 Mar 25;36(2):215-218. doi: 10.16250/j.32.1374.2023212. Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi. 2024. PMID: 38857969 Chinese.
-
Clinical use of polymerase chain reaction performed on peripheral blood and bone marrow samples for the diagnosis and monitoring of visceral leishmaniasis in HIV-infected and HIV-uninfected patients: a single-center, 8-year experience in Italy and review of the literature.Clin Infect Dis. 2007 Jun 15;44(12):1602-10. doi: 10.1086/518167. Epub 2007 May 7. Clin Infect Dis. 2007. PMID: 17516404 Review.
-
Diagnosis of canine visceral leishmaniasis: biotechnological advances.Vet J. 2008 Jan;175(1):45-52. doi: 10.1016/j.tvjl.2006.10.019. Epub 2006 Dec 5. Vet J. 2008. PMID: 17150389 Review.
References
-
- Chang L., Che G., Yang Q., Lai S., Teng J., Duan J., et al. . (2023). Leishmania donovani visceral leishmaniasis diagnosed by metagenomics next-generation sequencing in an infant with acute lymphoblastic leukemia: a case report. Front. Public Health 11. doi: 10.3389/fpubh.2023.1197149 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

