Efficacy and safety of paclitaxel drug-coated balloon angioplasty for stenosis of hemodialysis vascular access: 6-month results from a brazilian multicenter prospective study
- PMID: 39981420
- PMCID: PMC11841609
- DOI: 10.1590/1677-5449.202401032
Efficacy and safety of paclitaxel drug-coated balloon angioplasty for stenosis of hemodialysis vascular access: 6-month results from a brazilian multicenter prospective study
Abstract
Background: Stenosis resulting from neointimal hyperplasia remains a significant concern associated with dysfunction of arteriovenous fistulas (AVF).
Objectives: To investigate the safety and efficacy of paclitaxel drug-coated balloon (DCB) angioplasty for treating failing AVFs.
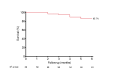
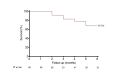
Methods: Investigators analyzed 58 hemodialysis patients treated with RangerTM DCBs from December 2022 to December 2023 across four centers. Lesions treated were de novo or restenotic and located in the juxta-anastomosis, cannulation zone, and outflow segment. Patients were evaluated through physical examinations and Duplex ultrasound at 1, 3, and 6 months. The primary efficacy endpoint was target lesion primary patency at 1, 3, and 6 months, and the primary safety endpoint was freedom from serious adverse events through 30 days post-procedure. Secondary endpoints were access circuit primary patency and technical and procedural success.
Results: Nine patients (16%) had thrombosed access at the initial presentation, and 31 (53%) presented with recurrent stenosis. The target lesion primary patency rate at 6 months was 85.7%, and the access circuit primary patency rate at 6 months was 67.5%. No serious adverse events, either local or systemic, were reported. Sex, age, stenosis location, type of lesion, presence of thrombosis, lesion recurrence, diabetes status, or whether post-ballooning dilation was performed did not significantly affect the 6-month target lesion primary patency.
Conclusions: DCB angioplasty was shown to be safe and effective for treating peripheral stenosis in vascular access.
Contexto: A estenose resultante da hiperplasia neointimal continua sendo uma preocupação significativa associada à disfunção das fístulas arteriovenosas (FAVs).
Objetivos: Investigar a segurança e eficácia da angioplastia com balão revestido com paclitaxel (DCB) para o tratamento de FAVs em falência.
Métodos: Os investigadores analisaram 58 pacientes em hemodiálise tratados com o DCB Ranger™ de dezembro de 2022 a dezembro de 2023, em quatro centros. As lesões tratadas eram de novo ou restenóticas, localizadas próximas à anastomose, na zona de canulação ou no segmento de saída. Os pacientes foram avaliados por meio de exames físicos e ultrassom Duplex aos 1, 3 e 6 meses. O desfecho primário de eficácia foi a perviedade primária da lesão-alvo aos 1, 3 e 6 meses, e o desfecho primário de segurança foi a ausência de eventos adversos graves até 30 dias após o procedimento. Os desfechos secundários foram a perviedade primária do circuito de acesso e o sucesso técnico e procedimental.
Resultados: Nove pacientes (16%) apresentaram trombose das FAVs na apresentação inicial e 31 (53%) apresentaram estenose recorrente. A taxa de perviedade primária da lesão-alvo aos 6 meses foi de 85,7%, e a taxa de perviedade primária do circuito de acesso aos 6 meses foi de 67,5%. Nenhum evento adverso grave, local ou sistêmico, foi relatado. Sexo, idade, localização da estenose, tipo de lesão, presença de trombose, recorrência da lesão, status diabético e realização de dilatação pós-balão não afetaram significativamente a perviedade primária da lesão-alvo aos 6 meses.
Conclusões: A angioplastia com DCB demonstrou ser segura e eficaz para o tratamento da estenose periférica em acessos vasculares.
Keywords: RangerTM; hemodialysis; paclitaxel-coated balloon; stenosis; vascular access.
Copyright© 2025 The authors.
Conflict of interest statement
Conflicts of interest: No conflicts of interest declared concerning the publication of this article.
Figures

References
-
- Lok CE, Huber TS, Lee T, et al. KDOQI clinical practice guideline for vascular access: 2019 update. [cited 2023 July 29];Am J Kidney Dis. 2020 75(4) suppl 2:S1–64. https://www.ajkd.org/article/S0272-6386(19)31137-0/fulltext . - PubMed
LinkOut - more resources
Full Text Sources
