17β-estradiol and estrogen receptor alpha protect mouse ovarian follicle development by repressing atresia
- PMID: 39981520
- PMCID: PMC11841210
- DOI: 10.1016/j.isci.2025.111846
17β-estradiol and estrogen receptor alpha protect mouse ovarian follicle development by repressing atresia
Abstract
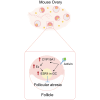
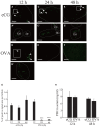
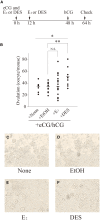
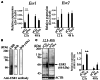
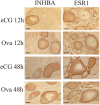
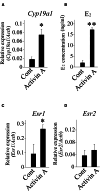
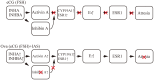
Mice administered an inhibin antiserum, equine chorionic gonadotropin (eCG) mixture called CARD HyperOva (OVA)/human chorionic gonadotropin (hCG), ovulate more oocytes than those administered eCG/hCG. In this study, the mechanism by which more oocytes are ovulated was investigated. Apoptotic cells were not observed in ovaries 24 and 48 h after OVA injection, and INHBA expression was absent in the growing follicles with apoptotic cells, whereas granulosa cells in follicles expressing INHBA also expressed estrogen receptor α (ESR1). ESR1 and CYP19A1 expression and 17β-estradiol (E2) in sera significantly increased in OVA-injected mice. Esr1 and Cyp19a1 expression and E2 concentration increased in ovaries cultured with activin A. The ovulation number increased in mice administered diethylstilbestrol. Taken together, these results suggest that ESR1 and E2 are involved in the inhibition of follicular atresia, which increases ovulation and oocyte number. This study may provide valuable information on the molecular mechanisms underlying mouse follicle selection.
Keywords: Endocrinology; Molecular physiology; Rodent reproduction.
© 2025 The Authors.
Conflict of interest statement
The authors declare no competing interests.
Figures



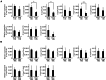
References
LinkOut - more resources
Full Text Sources
Miscellaneous

