Establishment and maintenance of embryogenic cell fate during microspore embryogenesis
- PMID: 39981724
- PMCID: PMC11843592
- DOI: 10.1111/tpj.17243
Establishment and maintenance of embryogenic cell fate during microspore embryogenesis
Abstract
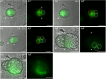
Microspore embryogenesis is a type of in vitro totipotency in which the immature male gametophyte (pollen) develops into a haploid embryo after an abiotic stress treatment. In Brassica napus, heat-stress treatment of male gametophytes induces the development of different types of multicellular embryogenic structures, each with different cellular characteristics and the capacity to form a differentiated embryo. The origin and early development of these different embryogenic structures have not been determined. We used two-photon excitation fluorescence microscopy and time-lapse imaging of cells expressing either a LEAFY COTYLEDON1 (LEC1) embryo identity reporter or a DR5v2 auxin response reporter to follow the development of embryogenic structures starting at the single- to few-cell stage. We show for the first time that the developmental fate of embryogenic structures is defined by the symmetry of the first embryogenic division and that the division plane also predicts the timing of subsequent pollen wall (exine) rupture: suspensorless embryos develop after a symmetric division and undergo late exine rupture, while suspensor-bearing embryos and embryogenic callus develop after an asymmetric division and undergo early exine rupture. Live imaging also captured previously unknown dynamic LEC1 and DR5v2 expression patterns that are associated with changes in exine integrity. This study highlights the developmental plasticity of cultured pollen and uncovers new roles for the first embryogenic cell division plane and the exine in defining and maintaining cell fate during microspore embryogenesis.
Keywords: Brassica napus; DR5v2; LEAFY COTYLEDON1; auxin; cell division plane; cell fate; microspore embryogenesis; totipotency.
© 2025 The Author(s). The Plant Journal published by Society for Experimental Biology and John Wiley & Sons Ltd.
Conflict of interest statement
The authors have not declared a conflict of interest.
Figures






References
-
- Corral‐Martínez, P. , Parra‐Vega, V. & Seguí‐Simarro, J.M. (2013) Novel features of Brassica napus embryogenic microspores revealed by high pressure freezing and freeze substitution: evidence for massive autophagy and excretion‐based cytoplasmic cleaning. Journal of Experimental Botany, 64, 3061–3075. - PubMed
-
- Corral‐Martínez, P. , Siemons, C. , Horstman, A. , Angenent, G.C. , de Ruijter, N. & Boutilier, K. (2020) Live imaging of embryogenic structures in Brassica napus microspore embryo cultures highlights the developmental plasticity of induced totipotent cells. Plant Reproduction, 33, 143–158. - PMC - PubMed
-
- Custers, J. (2003) Microspore culture in rapeseed (Brassica napus L.). In: Maluszynski, M. , Kasha, K.J. , Forster, B.P. & Szarejko, I. (Eds.) Doubled haploid production in crop plants. Dordrecht: Kluwer Academic Publishers, pp. 185–193.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

