Electronically Perturbed Vibrational Excitations of the Luminescing Stable Blatter Radical
- PMID: 39981951
- PMCID: PMC11887450
- DOI: 10.1021/acsnano.4c09661
Electronically Perturbed Vibrational Excitations of the Luminescing Stable Blatter Radical
Abstract
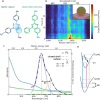
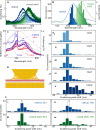
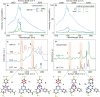
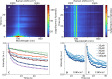
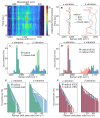
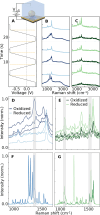
Stable radicals are spin-active species with a plethora of proposed applications in fields from energy storage and molecular electronics to quantum communications. However, their optical properties and vibrational modes are so far not well understood. Furthermore, it is not yet clear how these are affected by the radical oxidation state, which is key to understanding their electronic transport. Here, we identify the properties of 1,2,4-benzotriazin-4-yl, a stable doubly thiolated variant of the Blatter radical, using surface-enhanced Raman scattering (SERS). Embedding molecular monolayers in plasmonic nanocavities gives access to their vibrational modes, photoluminescence, and optical response during redox processes. We reveal the influence of the adjacent metallic surfaces and identify fluctuating SERS signals that suggest a coupling between the unpaired radical electron and a spatially overlapping vibrational mode. This can potentially be exploited for information-storage devices and chemically designed molecular qubits.
Keywords: Raman spectroscopy; SAM; SERS; electrochemistry; nanoparticles; nanophotonics; radicals.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
References
-
- Chen Z. X.; Li Y.; Huang F. Persistent and Stable Organic Radicals: Design, Synthesis, and Applications. Chem. 2021, 7 (2), 288–332. 10.1016/j.chempr.2020.09.024. - DOI
-
- Constantinides C. P.; Koutentis P. A. Stable N- and N/S-Rich Heterocyclic Radicals: Synthesis and Applications. Adv. Heterocycl. Chem. 2016, 119, 173–207. 10.1016/bs.aihch.2016.03.001. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

