Fluoxetine Treatment in Epilepsy of Infancy with Migrating Focal Seizures Due to KCNT1 Variants: An Open Label Study
- PMID: 39981956
- PMCID: PMC12174750
- DOI: 10.1002/ana.27213
Fluoxetine Treatment in Epilepsy of Infancy with Migrating Focal Seizures Due to KCNT1 Variants: An Open Label Study
Abstract
Objective: Gain-of-function (GoF) variants in KCNT1 encoding for potassium channels are associated with different epilepsy phenotypes, including epilepsy of infancy with migrating focal seizures (EIMFS), other early infantile developmental and epileptic encephalopathies, and focal epilepsy. Fluoxetine blocks currents from both wild-type (WT) and mutant KCNT1 channels with GoF in vitro features. In this study, we tested the hypothesis that treatment with fluoxetine might improve clinical outcome in patients with EIMFS carrying GoF variants in KCNT1 channels showing in vitro sensitivity to fluoxetine blockade.
Methods: We enrolled three pediatric patients carring de novo KCNT1 genetic variants linked to EIMFS. Functional and pharmacological studies to assess fluoxetine's ability to counteract in vitro variant-induced functional effects were performed with patch-clamp electrophysiology on heterologous channel expression in mammalian Chinese hamster ovary cells. Neuropsychological assessment, electroencephalogram and seizure diary were evaluated at baseline and every 3 months during the study. Electrocardiography and blood levels of medications were monitored for safety.
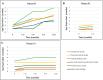
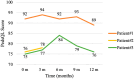
Results: All 3 KCNT1 variants displayed GoF effects in vitro. Exposure to fluoxetine (10μM) blocked both WT and mutant KCNT1 channels, therefore, counteracting variant-induced functional effects. Treatment with fluoxetine caused a variable reduction of seizure frequency (25-75%). Improvement in visual attention, participation, and muscle tone was also reported. No adverse events were reported except for transient dyskinesia in 1 patient, which was probably related to an increase in fluoxetine plasma level.
Interpretation: Fluoxetine may be a potential targeted medication in EIMFS caused by KCNT1 GoF variants. Further research is needed to assess its long-term efficacy and safety. ANN NEUROL 2025;98:48-61.
© 2025 The Author(s). Annals of Neurology published by Wiley Periodicals LLC on behalf of American Neurological Association.
Conflict of interest statement
Nothing to report.
Figures



References
MeSH terms
Substances
Grants and funding
- PNRR-MR1-2022-12376528/Italian Ministry for University and Research
- PRIN-PNRR 2022 P2022FJXY5/Italian Ministry for University and Research
- project MNESYS (PE0000006)/NEXTGENERATIONEU (NGEU), National Recovery and Resilience Plan (NRRP), - A Multiscale integrated approach to the study of the nervous system in health and disease
LinkOut - more resources
Full Text Sources
Medical

