The property of larval cells of the scleractinian coral, Acropora tenuis, deduced from in vitro cultured cells
- PMID: 39982014
- PMCID: PMC11997738
- DOI: 10.1111/dgd.70000
The property of larval cells of the scleractinian coral, Acropora tenuis, deduced from in vitro cultured cells
Abstract
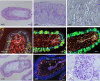
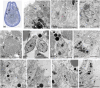
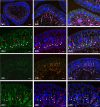
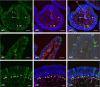
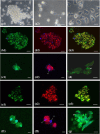
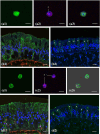
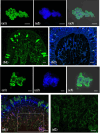
In previous studies, we have established approximately 15 cultured cell-lines derived from planula larvae of Acropora tenuis. Based on their morphology and behavior, these cells were classified into three types, flattened amorphous cells (FAmCs), vacuolated adherent cells (VAdCs), and small smooth cells (SSmCs). FAmCs include fibroblast-like cells and spherical, brilliant brown cells (BBrCs), which are transformable to each other. To examine the larval origin of the three cell types, we raised antibodies: anti-AtMLRP2 that appears to recognize FAmC, anti-AtAHNAK for BBrC, anti-AtSOMP5 and anti-AtEndoG for SSmC, and anti-AtGal and anti-AtFat4 for VAdC, respectively. Anti-AtMLRP2 antibody stained in vivo stomodeum and neuroblast-like cells embedded in larval ectoderm around the aboral pole. Anti-AtAHNAK antibody stained neuron-like and neuroblast-like cells, both of which were also stained with neuron-specific tubulin β-3 antibody. These results suggest that in vitro BBrCs and in vivo neuroblast-like cells share neuronal properties in common. Two antibodies for SSmCs, anti-AtSOMP5 and anti-AtEndoG, stained larval ectoderm cells, suggesting that SSmCs have larval ectoderm properties. Two antibodies for VAdCs, anti-AtGal and anti-AtFat4, stained larval endoderm cells, suggesting that VAdCs have larval endoderm properties. Therefore, the in vitro cell lines appear to retain properties of the stomodeum, neuroblast, ectoderm, or endoderm. Each of them may be used in future investigations to reveal cellular and molecular properties of cell types of coral larvae, such as the potential for symbiosis.
Keywords: Acropora coral; ectoderm; endoderm; in vitro cell system; larval cell property; neuroblast; stomodeum.
© 2025 The Author(s). Development, Growth & Differentiation published by John Wiley & Sons Australia, Ltd on behalf of Japanese Society of Developmental Biologists.
Conflict of interest statement
We declare no competing interests.
Figures


Similar articles
-
Establishing Sustainable Cell Lines of a Coral, Acropora tenuis.Mar Biotechnol (NY). 2021 Jun;23(3):373-388. doi: 10.1007/s10126-021-10031-w. Epub 2021 Apr 26. Mar Biotechnol (NY). 2021. PMID: 33899125 Free PMC article.
-
Chromera velia is endosymbiotic in larvae of the reef corals Acropora digitifera and A. tenuis.Protist. 2013 Mar;164(2):237-44. doi: 10.1016/j.protis.2012.08.003. Epub 2012 Oct 12. Protist. 2013. PMID: 23063731
-
The Mesoderm-Forming Gene brachyury Regulates Ectoderm-Endoderm Demarcation in the Coral Acropora digitifera.Curr Biol. 2016 Nov 7;26(21):2885-2892. doi: 10.1016/j.cub.2016.08.011. Epub 2016 Sep 29. Curr Biol. 2016. PMID: 27693135
-
Embryonic Development of the Gastrodermis in the Coral Acropora tenuis.Zoolog Sci. 2024 Dec;41(6):496-508. doi: 10.2108/zs240032. Zoolog Sci. 2024. PMID: 39636132
-
In Vitro Phagocytosis of Different Dinoflagellate Species by Coral Cells.Zoolog Sci. 2023 Dec;40(6):444-454. doi: 10.2108/zs230045. Zoolog Sci. 2023. PMID: 38064371
References
-
- Attenborough, R. M. F. , Hayward, D. C. , Wiedemann, U. , Forêt, S. , Miller, D. J. , & Ball, E. E. (2019). Expression of the neuropeptides RFamide and LWamide during development of the coral *Acropora millepora* in relation to settlement and metamorphosis. Developmental Biology, 446, 56–67. - PubMed
-
- Bode, H. R. , Heimfeld, S. , Chow, M. A. , & Huang, L. W. (1987). Gland cells arise by differentiation from interstitial cells in Hydra attenuata . Developmental Biology, 122, 577–585. - PubMed
-
- Chapman, D. M. (1999). Microanatomy of the bell rim of Aurelia aurita (cnidaria: Scyphozoa). Canadian Journal of Zoology, 77, 34–46.
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

