A link between aging and persistence
- PMID: 39982072
- PMCID: PMC11963536
- DOI: 10.1128/aac.01313-24
A link between aging and persistence
Abstract
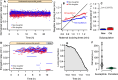
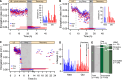
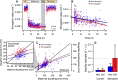
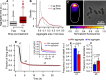
Despite the various strategies that microorganisms have evolved to resist antibiotics, survival to drug treatments can be driven by subpopulations of susceptible bacteria in a transient state of dormancy. This phenotype, known as bacterial persistence, arises due to a natural and ubiquitous heterogeneity of growth states in bacterial populations. Nonetheless, the unifying mechanism of persistence remains unknown, with several pathways being able to trigger the phenotype. Here, we show that asymmetric damage partitioning, a form of cellular aging, produces the underlying phenotypic heterogeneity upon which persistence is triggered. Using single-cell microscopy and microfluidic devices, we demonstrate that deterministic asymmetry in exponential phase populations leads to a state of growth stability, which prevents the spontaneous formation of persisters. However, as populations approach stationary phase, aging bacteria-those inheriting more damage upon division-exhibit a sharper growth rate decline, increased probability of growth arrest, and higher persistence rates. These results indicate that persistence triggers are biased by bacterial asymmetry, thus acting upon the deterministic heterogeneity produced by cellular aging. This work suggests unifying mechanisms for persistence and offers new perspectives on the treatment of recalcitrant infections.
Keywords: Escherichia coli; aging; microfluidics; persistence.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Hobby GL, Meyer K, Chaffee E. 1942. Observations on the mechanism of action of penicillin. Exp Biol Med (Maywood) 50:281–285. doi:10.3181/00379727-50-13773 - DOI
-
- Balaban Nathalie Q, Helaine S, Lewis K, Ackermann M, Aldridge B, Andersson DI, Brynildsen MP, Bumann D, Camilli A, Collins JJ, Dehio C, Fortune S, Ghigo J-M, Hardt W-D, Harms A, Heinemann M, Hung DT, Jenal U, Levin BR, Michiels J, Storz G, Tan M-W, Tenson T, Van Melderen L, Zinkernagel A. 2019. Definitions and guidelines for research on antibiotic persistence. Nat Rev Microbiol 17:441–448. doi:10.1038/s41579-019-0196-3 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

