Development of a Core Critical Care Data Dictionary With Common Data Elements to Characterize Critical Illness and Injuries Using a Modified Delphi Method
- PMID: 39982128
- PMCID: PMC12047641
- DOI: 10.1097/CCM.0000000000006595
Development of a Core Critical Care Data Dictionary With Common Data Elements to Characterize Critical Illness and Injuries Using a Modified Delphi Method
Abstract
Objectives: To develop the first core Critical Care Data Dictionary (C2D2) with common data elements (CDEs) to characterize critical illness and injuries.
Design: Group consensus process using modified Delphi approach.
Setting: Electronic surveys and in-person meetings.
Subjects: A multidisciplinary workgroup of clinicians and researchers with expertise in the care of the critically ill and injured.
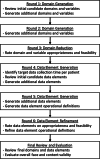
Interventions: The Delphi process was divided into domain and CDE portions with each composed of two item generation rounds and one item reduction/refinement rounds. Two in-person meetings augmented this process to facilitate review and consideration of the domains and by panel members. The final set of domains and CDEs was then reviewed by the group to meet the competing criteria of utility and feasibility, resulting in the core dataset.
Measurements and main results: The 23-member Delphi panel was provided 1833 candidate variables for potential dataset inclusion. The final dataset includes 226 patient-level CDCs in nine domains, which include anthropometrics and demographics (8), chronic comorbid illnesses (18), advanced directives (1), ICU diagnoses (61), diagnostic tests (42), interventions (27), medications (38), objective assessments (26), and hospital course and outcomes (5). Upon final review, 91% of the panel endorsed the CDCs as meeting criteria for a minimum viable data dictionary. Data elements cross the lifespan of neonate through adult patients.
Conclusions: The resulting C2D2 provides a foundation to facilitate rapid collection, analyses, and dissemination of information necessary for research, quality improvement, and clinical practice to optimize critical care outcomes. Further work is needed to validate the effectiveness of the dataset in a variety of critical care settings.
Keywords: adult; common data elements; intensive care; neonates; pediatric; standard definitions.
Copyright © 2025 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the Society of Critical Care Medicine and Wolters Kluwer Health, Inc.
Conflict of interest statement
Drs. Murphy’s and Sikora’s institutions received funding from the Agency for Health Care Research and Quality. Dr. Murphy’s institution received funding from the Centers for Disease Control and Prevention. Dr. Cruz-Cano’s institution received funding from the Society of Critical Care Medicine (SCCM). Dr. Kamaleswaran’s institution received funding from the National Institutes of Health (NIH). Drs. Kamaleswaran, Sikora, Tanner, and Wong received support for article research from the NIH. Dr. Badawi received funding from Ceiba Healthcare and CLEW Medical. Dr. Khanna received funding from Medtronic, Edwards Life Sciences, Philips Research North America, GE Healthcare, Retia Medical, Caretaker Medical, BrainX, the Canadian Institutes of Health Research, the Department of Defense, the National Heart, Lung, and Blood Institute, and the National Center for Advancing Translational Sciences. Dr. Rincon received funding from Blue Cirrus Consulting, Baxter Health Consulting, Viven Health, Phillips Health, Hospital Insurance Forum, and Illinois Health and Hospital Association. Drs. Wong, Zimmerman, and Cobb received funding from the SCCM. Dr. Wong received funding from Ataia Medical. Dr. Wynn received funding from Sobi. Dr. Zhang received support for article research from the SCCM. Dr. Zimmerman’s institution received funding from the National Institute of Child Health and Human Development and Immunexpress. Drs. Zimmerman and Reuter-Rice received funding from Elsevier Publishing. Dr. Cobb received funding from Giblib, Akido Labs, and Bauhealth. Dr. Reuter-Rice’s institution received funding from the National Institute of Neurological Disorders and Stroke; she received funding from Delphi. The remaining authors have disclosed that they do not have any potential conflicts of interest.
Figures
References
-
- Angus DC, Barnato AE, Linde-Zwirble WT, et al. ; Robert Wood Johnson Foundation ICU End-Of-Life Peer Group: Use of intensive care at the end of life in the United States: An epidemiologic study. Crit Care Med. 2004; 32:638–643 - PubMed
-
- Deutschman CS, Ahrens T, Cairns CB, et al. ; Critical Care Societies Collaborative USCIITG Task Force on Critical Care Research: Multisociety task force for critical care research: Key issues and recommendations. Crit Care Med. 2012; 40:254–260 - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical