Propranolol As an Anxiolytic to Reduce the Use of Sedatives for Critically Ill Adults Receiving Mechanical Ventilation (PROACTIVE): An Open-Label Randomized Controlled Trial
- PMID: 39982178
- PMCID: PMC11801419
- DOI: 10.1097/CCM.0000000000006534
Propranolol As an Anxiolytic to Reduce the Use of Sedatives for Critically Ill Adults Receiving Mechanical Ventilation (PROACTIVE): An Open-Label Randomized Controlled Trial
Abstract
Objectives: Surges in demand for sedatives for mechanical ventilation during the COVID-19 pandemic caused shortages of sedatives globally. Propranolol, a nonselective beta-adrenergic blocker, has been associated with reduced agitation and sedative needs in observational studies. We aimed to test whether propranolol could reduce the dose of sedatives needed in mechanically ventilated patients.
Design: Open-label randomized controlled trial.
Setting: Three academic hospitals.
Subjects: Any nonparalyzed patient receiving mechanical ventilation and requiring high-dose sedatives.
Interventions: Enteral propranolol 20-60 mg every 6 hours titrated to effect in the intervention group; all participants received protocol-titrated sedation with propofol or midazolam.
Measurements and main results: Mean change in 24 hours dose of sedative from baseline to day 3, proportion of sedation scores within target, and occurrence rate of adverse events. We enrolled a planned 72 patients between January 2021 and October 2022. Sixty-nine percent were male with a mean (sd) age of 54 years (15.91 yr). Most were admitted for COVID or non-COVID pneumonia. Intervention participants received propranolol for a mean of 10 days (mean daily dose, 90 mg). There was a significantly larger decrease in sedative dose from baseline (54% vs. 34%; p = 0.048) and more sedation assessments within target range (48% vs. 35%; p < 0.0001) in the intervention group compared with controls. There were no differences in mortality or adverse events.
Conclusions: Propranolol is an inexpensive drug that effectively lowered the need for sedatives in critically ill patients managed in the COVID-19 pandemic. Propranolol may help preserve limited supplies of sedatives while achieving target sedation.
Trial registration: ClinicalTrials.gov NCT04467086.
Copyright © 2025 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the Society of Critical Care Medicine and Wolters Kluwer Health, Inc.
Conflict of interest statement
Dr. Downar, Ms. Lapenskie, Dr. Kanji, Ms. Saeed, Mr. Anderson, and Dr. Fox-Robichaud disclosed off-label use of propranolol. Ms. Lapenskie’s, Ms. Saeed’s, Dr. Polskaia’s, Mr. Anderson’s, and Dr. Fox-Robichaud’s institutions received funding from the Hamilton Academic Health Sciences Organization (HAHSO). Ms. Lapenskie, Ms. Saeed, Dr. Polskaia, and Mr. Anderson received support for article research from the HAHSO. Ms. Haines received support for article research from the Canadian Institutes of Health Research (CIHR); she disclosed government work. Ms. Porteous received support for article research from Hamilton Health. Dr. Fox-Robichaud’s institution received funding from the CIHR; she received funding from Hamilton Health Sciences and McMaster University. The remaining authors have disclosed that they do not have any potential conflicts of interest.
Figures
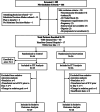

References
-
- Devlin JW, Skrobik Y, Gelinas C, et al. : Executive summary: Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med. 2018; 46:1532–1548 - PubMed
-
- Drugshortagescanada.ca: Propofol Shortage Reports. Available at: https://www.drugshortagescanada.ca/search?term=propofol&date_property=up.... Accessed June 1, 2021
-
- Drugshortagescanada.ca: Midazolam Shortage Reports. Available at: https://www.drugshortagescanada.ca/search?term=midazolam&date_property=u.... Accessed June 1, 2021
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

