Short Duration of Antenatal Corticosteroid Exposure and Outcomes in Extremely Preterm Infants
- PMID: 39982720
- PMCID: PMC11846007
- DOI: 10.1001/jamanetworkopen.2024.61312
Short Duration of Antenatal Corticosteroid Exposure and Outcomes in Extremely Preterm Infants
Abstract
Importance: When preterm delivery is imminent, it remains unclear whether the timing from administration of antenatal betamethasone to birth may reduce mortality and morbidity among extremely preterm infants.
Objective: To evaluate the association of duration from exposure to first dose of antenatal betamethasone with outcomes among extremely preterm infants.
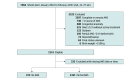
Design, setting, and participants: This cohort study enrolled infants born at 22 0/7 to 27 6/7 weeks' gestation from January 2016 to February 2021 at National Institute of Child Health and Human Development Neonatal Research Network centers. Infants exposed to multiple doses of antenatal betamethasone, infants who did not receive intensive care, and infants with congenital anomalies were excluded. Data were analyzed from October 2021 to December 2024.
Exposure: Time in hours from anenatal betamethasone administration to birth.
Main outcomes and measures: The primary outcome was survival to discharge. Secondary outcomes included survival without major morbidity and composites of individual morbidities and death. The association of time from antenatal betamethasone administration to birth with neonatal survival and morbidity was assessed using generalized linear models, adjusting for gestational age, infant sex, maternal race, education, small for gestational age, mode of delivery, multiple birth, prolonged rupture of membranes, and center of birth.
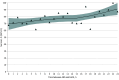
Results: Of 7464 infants born during the study period, 1806 infants (928 [51.3%] boys) were included in the cohort: 475 with no betamethasone and 1331 with exposure to a single dose of betamethasone within 24 hours before birth. The median (IQR) administration-to-birth interval for infants born after a single dose of betamethasone was 3.8 (1.4-9.5) hours. The administration-to-birth interval was independently associated with survival (adjusted relative risk [aRR] per 1-hour increase, 1.01 [95% CI, 1.00-1.01]; aRR per 6-hour increase, 1.04 [95% CI, 1.01-1.07]) and survival without severe neonatal morbidity (aRR per 1-hour increase, 1.01 [95% CI, 1.01-1.02]; aRR per 6-hour increase, 1.09 [95% CI, 1.04-1.14].
Conclusions and relevance: In this cohort study, for women at risk of imminent preterm birth, even short duration of exposure to antenatal betamethasone was associated with improved neonatal survival and survival without severe neonatal morbidity.
Conflict of interest statement
Figures
References
-
- ACOG Committee Opinion No . ACOG committee opinion No. 475: antenatal corticosteroid therapy for fetal maturation. Obstet Gynecol. 2011;117(2 Pt 1):422-424. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

