A hormone-to-neuropeptide pathway inhibits sexual receptivity in immature Drosophila females
- PMID: 39982743
- PMCID: PMC11874258
- DOI: 10.1073/pnas.2418481122
A hormone-to-neuropeptide pathway inhibits sexual receptivity in immature Drosophila females
Abstract
Newborns, typically asexual, undergo a process of sexual transition to reach sexual maturity, but the regulatory mechanism underlying this transition is not clear. Here, we studied how female sexual behavior is modulated during sexual transition by hormones and neuromodulators in Drosophila. We found that neuropeptide Leucokinin (LK) inhibits female receptivity specifically during a sexual transition period in immature females, but not in younger or mature females. Moreover, the steroid hormone ecdysone, which is mainly synthesized in the female ovary during sexual maturation, acts on LK neurons via the ecdysone receptor to suppress sexual receptivity. We further found that LK suppresses female receptivity through its receptor LKR in central pC1 neurons, a decision center for female sexual behavior. These findings reveal a hormone-to-neuropeptide pathway that specifically inhibits sexual behavior during sexual maturation in female Drosophila, shedding light on how hormones and neuromodulators coordinate sexual development and behaviors.
Keywords: ecdysone; female receptivity; juvenile hormone; neuropeptide; sexual transition.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures
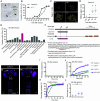

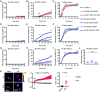



Similar articles
-
The function of juvenile-adult transition axis in female sexual receptivity of Drosophila melanogaster.Elife. 2024 Sep 6;12:RP92545. doi: 10.7554/eLife.92545. Elife. 2024. PMID: 39240259 Free PMC article.
-
Inter-organ steroid hormone signaling promotes myoblast fusion via direct transcriptional regulation of a single key effector gene.Curr Biol. 2024 Apr 8;34(7):1438-1452.e6. doi: 10.1016/j.cub.2024.02.056. Epub 2024 Mar 20. Curr Biol. 2024. PMID: 38513654 Free PMC article.
-
Neuropeptide-mediated synaptic plasticity regulates context-dependent mating behaviors in Drosophila.PLoS Biol. 2025 Sep 4;23(9):e3003330. doi: 10.1371/journal.pbio.3003330. eCollection 2025 Sep. PLoS Biol. 2025. PMID: 40906816 Free PMC article.
-
Ovarian hormones and obesity.Hum Reprod Update. 2017 May 1;23(3):300-321. doi: 10.1093/humupd/dmw045. Hum Reprod Update. 2017. PMID: 28333235 Free PMC article. Review.
-
Pharmacological interventions for those who have sexually offended or are at risk of offending.Cochrane Database Syst Rev. 2015 Feb 18;2015(2):CD007989. doi: 10.1002/14651858.CD007989.pub2. Cochrane Database Syst Rev. 2015. PMID: 25692326 Free PMC article.
Cited by
-
Distributed control circuits across a brain-and-cord connectome.bioRxiv [Preprint]. 2025 Aug 2:2025.07.31.667571. doi: 10.1101/2025.07.31.667571. bioRxiv. 2025. PMID: 40766407 Free PMC article. Preprint.
-
Behavior choices amongst grooming, feeding, and courting in Drosophila show contextual flexibility, not an absolute hierarchy of needs.bioRxiv [Preprint]. 2025 May 13:2025.05.09.653186. doi: 10.1101/2025.05.09.653186. bioRxiv. 2025. PMID: 40463182 Free PMC article. Preprint.
-
Mating status-dependent dopaminergic modulation of auditory sensory neurons in Drosophila.iScience. 2025 Jul 29;28(9):113232. doi: 10.1016/j.isci.2025.113232. eCollection 2025 Sep 19. iScience. 2025. PMID: 40831745 Free PMC article.
References
-
- Ellison P. T., et al. , Puberty as a life history transition. Ann. Hum. Biol. 39, 352–360 (2012). - PubMed
-
- Phoenix C. H., Goy R. W., Gerall A. A., Young W. C., Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology 65, 369–382 (1959). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

