Culture conditions differentially regulate the inflammatory niche and cellular phenotype of tracheobronchial basal stem cells
- PMID: 39982813
- PMCID: PMC12261264
- DOI: 10.1152/ajplung.00293.2024
Culture conditions differentially regulate the inflammatory niche and cellular phenotype of tracheobronchial basal stem cells
Abstract
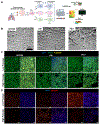
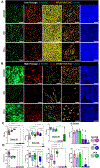
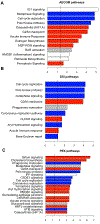
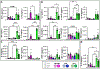
Bronchial epithelial cells derived from the tracheobronchial regions of human airways (HBECs) provide a valuable in vitro model for studying pathological mechanisms and evaluating therapeutics. This cell population comprises a mixed population of basal cells (BCs), the predominant stem cell in airways capable of both self-renewal and functional differentiation. Despite their potential for regenerative medicine, BCs exhibit significant phenotypic variability in culture. To investigate how culture conditions influence BC phenotype and function, we expanded three independent BC isolates in three media: airway epithelial cell growth medium (AECGM), dual-SMAD inhibitor (DSI)-enriched AECGM, and PneumaCult Ex plus (PEx+). Analysis through RNA sequencing, immune assays, and impedance measurements revealed that PEx+ media significantly drove cell proliferation and a broad proinflammatory phenotype in BCs. In contrast, BCs expanded in AECGM and displayed increased expression of structural and extracellular matrix components at higher passage. AECGM increased expression of some cytokines at high passage, whereas DSI suppressed inflammation implicating the involvement TGF-β in BC inflammatory processes. Differentiation capacity of BCs declined with time in culture irrespective of expansion media. This was associated with an increase in PLUNC expressing secretory cells in AECGM and PEx+ media consistent with the known immune modulatory role of PLUNC in the airways. These findings highlight the profound impact of media conditions on inflammatory niche established by, and function of, in vitro expanded BCs. The broad proinflammatory phenotype driven by PEx+ media, in particular, should be considered in the development of cell-based models for airway diseases and therapeutic applications.NEW & NOTEWORTHY Airway basal cells, vital for airway regeneration and potential therapies, show significant changes based on culture conditions. Our study reveals that media composition and culture duration greatly affect basal cell properties with profound changes in the proinflammatory phenotype and extracellular matrix deposition driven by changes in growth conditions. These results underscore the critical impact of culture conditions on BC phenotype, influencing cell-based models for airway disease research and therapy.
Keywords: RNA sequencing; airway epithelium; cell proliferation; differentiation; inflammatory niche.
Conflict of interest statement
DISCLOSURES
At the time of submission, the authors have no perceived or potential conflict of interest, financial or otherwise to disclose.
Figures



Update of
-
Culture Conditions Differentially Regulate the Inflammatory Niche and Cellular Phenotype of Tracheo-Bronchial Basal Stem Cells.bioRxiv [Preprint]. 2024 Sep 5:2024.09.04.611264. doi: 10.1101/2024.09.04.611264. bioRxiv. 2024. Update in: Am J Physiol Lung Cell Mol Physiol. 2025 Apr 01;328(4):L538-L553. doi: 10.1152/ajplung.00293.2024. PMID: 39282256 Free PMC article. Updated. Preprint.
Similar articles
-
Culture Conditions Differentially Regulate the Inflammatory Niche and Cellular Phenotype of Tracheo-Bronchial Basal Stem Cells.bioRxiv [Preprint]. 2024 Sep 5:2024.09.04.611264. doi: 10.1101/2024.09.04.611264. bioRxiv. 2024. Update in: Am J Physiol Lung Cell Mol Physiol. 2025 Apr 01;328(4):L538-L553. doi: 10.1152/ajplung.00293.2024. PMID: 39282256 Free PMC article. Updated. Preprint.
-
Airway epithelial stem cell renewal and differentiation: overcoming challenging steps towards clinical-grade tissue engineering.Stem Cell Res Ther. 2025 Jul 6;16(1):351. doi: 10.1186/s13287-025-04478-0. Stem Cell Res Ther. 2025. PMID: 40619403 Free PMC article.
-
Culture and expansion of murine proximal airway basal stem cells.Stem Cell Res Ther. 2024 Jan 30;15(1):26. doi: 10.1186/s13287-024-03642-2. Stem Cell Res Ther. 2024. PMID: 38287366 Free PMC article.
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
-
Computer and mobile technology interventions for self-management in chronic obstructive pulmonary disease.Cochrane Database Syst Rev. 2017 May 23;5(5):CD011425. doi: 10.1002/14651858.CD011425.pub2. Cochrane Database Syst Rev. 2017. PMID: 28535331 Free PMC article.
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

