A single dose of inactivated influenza virus vaccine expressing COBRA hemagglutinin elicits broadly-reactive and long-lasting protection
- PMID: 39982912
- PMCID: PMC11844911
- DOI: 10.1371/journal.pone.0308680
A single dose of inactivated influenza virus vaccine expressing COBRA hemagglutinin elicits broadly-reactive and long-lasting protection
Abstract
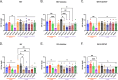
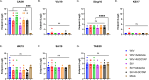
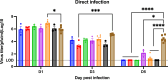
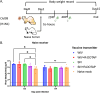
Influenza virus infections present a pervasive global health concern resulting in millions of hospitalizations and thousands of fatalities annually. To address the influenza antigenic variation, the computationally optimized broadly reactive antigen (COBRA) methodology was used to design influenza hemagglutinin (HA) or neuraminidase (NA) for universal influenza vaccine candidates. In this study, whole inactivated virus (WIV) or split inactivated virus (SIV) vaccine formulations expressing either the H1 COBRA HA or H3 COBRA HA were formulated with or without an adjuvant and tested in ferrets with pre-existing anti-influenza immunity. A single dose of the COBRA-WIV vaccine elicited a robust and broadly reactive antibody response against H1N1 and H3N2 influenza viruses. In contrast, the COBRA-SIV elicited antibodies that recognized fewer viruses, but with R-DOATP, its specificity was expanded. Vaccinated ferrets were protected against morbidity and mortality following challenge with A/California/07/2009 at 14 weeks post-vaccination with reduced viral shedding post-infection compared to the naïve ferrets. However, the COBRA-IIVs did not block the viral transmission to naïve ferrets. The contact infection induced less severe disease and delayed viral shedding than direct infection. Overall, the COBRA HA WIV or the COBRA HA SIV plus R-DOTAP elicited broadly reactive antibodies with long-term protection against viral challenge and reduced viral transmission following a single dose of vaccine in ferrets pre-immune to historical H1N1 and H3N2 influenza viruses. IMPORTANCE The goal of the next-generation influenza vaccine is to provide broadly reactive protection against various drifted influenza strains. With the previous studies evaluating the COBRA HA-based vaccines, the breadth of antibody activities was confirmed following two or three vaccinations. However, for the commercial influenza vaccine, only one shot is required. In this study, only one shot was administrated to the pre-immune ferrets and the COBRA-WIV efficiently elicited broadly reactive antibodies and long-lasting protection against the pdm09 strain. Moreover, this study showed that different infection methods can lead to different disease severity, which emphasizes the significance of the model selection. The infection was conducted 14 weeks post-vaccination to evaluate the long-term protection elicited by only one vaccination. This is the first longevity study describing the immune responses elicited by COBRA-IIVs in ferrets and provides promising results for the potential clinical utilization.
Copyright: © 2025 Shi et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




Similar articles
-
Elicitation of Protective Antibodies against 20 Years of Future H3N2 Cocirculating Influenza Virus Variants in Ferrets Preimmune to Historical H3N2 Influenza Viruses.J Virol. 2019 Jan 17;93(3):e00946-18. doi: 10.1128/JVI.00946-18. Print 2019 Feb 1. J Virol. 2019. PMID: 30429350 Free PMC article.
-
Elicitation of Protective Antibodies against a Broad Panel of H1N1 Viruses in Ferrets Preimmune to Historical H1N1 Influenza Viruses.J Virol. 2017 Nov 30;91(24):e01283-17. doi: 10.1128/JVI.01283-17. Print 2017 Dec 15. J Virol. 2017. Retraction in: J Virol. 2024 Jul 23;98(7):e0183223. doi: 10.1128/jvi.01832-23. PMID: 28978709 Free PMC article. Retracted.
-
Split inactivated COBRA vaccine elicits protective antibodies against H1N1 and H3N2 influenza viruses.PLoS One. 2018 Sep 28;13(9):e0204284. doi: 10.1371/journal.pone.0204284. eCollection 2018. PLoS One. 2018. PMID: 30265682 Free PMC article.
-
Correlates of protection against influenza infection in humans--on the path to a universal vaccine?Curr Opin Immunol. 2013 Aug;25(4):470-6. doi: 10.1016/j.coi.2013.07.005. Epub 2013 Aug 13. Curr Opin Immunol. 2013. PMID: 23948572 Review.
-
Pseudotype-based neutralization assays for influenza: a systematic analysis.Front Immunol. 2015 Apr 29;6:161. doi: 10.3389/fimmu.2015.00161. eCollection 2015. Front Immunol. 2015. PMID: 25972865 Free PMC article. Review.
References
-
- Wang X, Li Y, O’Brien KL, Madhi SA, Widdowson M-A, Byass P, et al.. Global burden of respiratory infections associated with seasonal influenza in children under 5 years in 2018: a systematic review and modelling study. The Lancet Global Health. 2020;8(4):e497–e510. doi: 10.1016/S2214-109X(19)30545-5 - DOI - PMC - PubMed
-
- Lafond KE, Porter RM, Whaley MJ, Suizan Z, Ran Z, Aleem MA, et al.. Global burden of influenza-associated lower respiratory tract infections and hospitalizations among adults: A systematic review and meta-analysis. PLoS Medicine. 2021;18(3):e1003550. doi: 10.1371/journal.pmed.1003550 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

