Supersulfides contribute to joint homeostasis and bone regeneration
- PMID: 39983344
- PMCID: PMC11893308
- DOI: 10.1016/j.redox.2025.103545
Supersulfides contribute to joint homeostasis and bone regeneration
Abstract
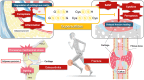
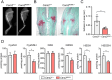
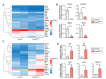
The physiological functions of supersulfides, inorganic and organic sulfides with sulfur catenation, have been extensively studied. Their synthesis is mainly mediated by mitochondrial cysteinyl-tRNA synthetase (CARS2) that functions as a principal cysteine persulfide synthase. This study aimed to investigate the role of supersulfides in joint homeostasis and bone regeneration. Using Cars2AINK/+ mutant mice, in which the KIIK motif of CARS2 essential for supersulfide production was replaced with AINK, we evaluated the role of supersulfides in fracture healing and cartilage homeostasis during osteoarthritis (OA). Tibial fracture surgery was performed on the wild-type (Cars2+/+) and Cars2AINK/+ mice littermates. Bulk RNA-seq analysis for the osteochondral regeneration in the fracture model showed increased inflammatory markers and reduced osteogenic factors, indicative of impaired bone regeneration, in Cars2AINK/+ mice. Destabilization of the medial meniscus (DMM) surgery was performed to produce the mouse OA model. Histological analyses with Osteoarthritis Research Society International and synovitis scores revealed accelerated OA progression in Cars2AINK/+ mice compared with that in Cars2+/+ mice. To assess the effects of supersulfides on OA progression, glutathione trisulfide (GSSSG) or saline was periodically injected into the mouse knee joints after the DMM surgery. Thus, supersulfides derived from CARS2 and GSSSG exogenously administered significantly inhibited inflammation and lipid peroxidation of the joint cartilage, possibly through suppression of ferroptosis, during OA development. This study represents a significant advancement in understanding anti-inflammatory and anti-oxidant functions of supersulfides in skeletal tissues and may have a clinical relevance for the bone healing and OA therapeutics.
Keywords: Bone regeneration; Cysteinyl-tRNA synthetase; Ferroptosis; Glutathione trisulfide; Osteoarthritis; Supersulfides.
Copyright © 2025 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no competing interests.
Figures


References
-
- Akaike T., Ida T., Wei F.Y., Nishida M., Kumagai Y., Alam M.M., Ihara H., Sawa T., Matsunaga T., Kasamatsu S., Nishimura A., Morita M., Tomizawa K., Nishimura A., Watanabe S., Inaba K., Shima H., Tanuma N., Jung M., Fujii S., Watanabe Y., Ohmuraya M., Nagy P., Feelisch M., Fukuto J.M., Motohashi H. Cysteinyl-tRNA synthetase governs cysteine polysulfidation and mitochondrial bioenergetics. Nat. Commun. 2017;8:1177. doi: 10.1038/s41467-017-01311-y. - DOI - PMC - PubMed
-
- Ida T., Sawa T., Ihara H., Tsuchiya Y., Watanabe Y., Kumagai Y., Suematsu M., Motohashi H., Fujii S., Matsunaga T., Yamamoto M., Ono K., Devarie-Baez N.O., Xian M., Fukuto J.M., Akaike T. Reactive cysteine persulfides and S-polythiolation regulate oxidative stress and redox signaling. Proc. Natl. Acad. Sci. U.S.A. 2014;111:7606–7611. doi: 10.1073/pnas.1321232111. - DOI - PMC - PubMed
-
- Fukuto J.M., Ignarro L.J., Nagy P., Wink D.A., Kevil C.G., Feelisch M., Cortese-Krott M.M., Bianco C.L., Kumagai Y., Hobbs A.J., Lin J., Ida T., Akaike T. Biological hydropersulfides and related polysulfides - a new concept and perspective in redox biology. FEBS Lett. 2018;592:2140–2152. doi: 10.1002/1873-3468.13090. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

