Changes in Liver Function Tests, Congestion, and Prognosis After Acute Heart Failure: The STRONG-HF Trial
- PMID: 39983609
- PMCID: PMC11891714
- DOI: 10.1016/j.jacadv.2025.101607
Changes in Liver Function Tests, Congestion, and Prognosis After Acute Heart Failure: The STRONG-HF Trial
Abstract
Background: Elevated alanine aminotransferase (ALT), aspartate aminotransferase (AST), and total bilirubin (tBil) may reflect congestion and liver dysfunction in acute heart failure (AHF), while lower ALT also associates with sarcopenia.
Objectives: The purpose of this study was to assess ALT, AST, and tBil levels in AHF patients during high-intensity care (HIC) vs usual care (UC) follow-up.
Methods: ALT, AST, and tBil were measured 1 to 2 days predischarge in 1,062 AHF patients, and again after 90 days of either HIC or UC according to the STRONG-HF (Safety, Tolerability and efficacy of Rapid Optimization, helped by NT-proBNP testinG, of Heart Failure therapies) protocol. The primary endpoint was 180-day all-cause death or HF hospitalization.
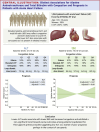
Results: Median (Q1-Q3) baseline ALT, AST, and tBil were 21 (15-32) U/L, 23 (17-32) U/L, and 14(10-21) umol/L, respectively. Patients with lower ALT had lower body mass index. Patients with lower ALT, but not tBil or AST, were more likely to have edema, elevated jugular venous pressure, and orthopnea, and use more diuretics prerandomization. A nonsignificant inverse association between ALT and the primary outcome (HR: 0.82 [95% CI: 0.66-1.01] per log-unit, P = 0.06) was observed. Greater reductions of ALT, AST, and tBil to 90 days were associated with greater improvement of edema, rales, NYHA functional class, and N-terminal pro-B-type natriuretic peptide. After 90 days, the HIC group had a greater reduction in AST and tBil than the UC group, while nonsignificant for ALT. The treatment effect of HIC over UC on the primary outcome was consistent across the baseline ALT, AST, and tBil range (all P interaction >0.10), but patients with lower ALT experienced greater health status improvement from HIC.
Conclusions: Lower ALT was associated with lower body mass index and more congestion in AHF, supporting previous studies suggesting ALT as a sarcopenia marker. The beneficial effect of HIC on health status was greater in low baseline ALT patients. (Safety, Tolerability and Efficacy of Rapid Optimization, Helped by NT-proBNP testinG, of Heart Failure Therapies [STRONG-HF]; NCT03412201).
Keywords: ALT; acute heart failure; high intensity care; liver function tests.
Copyright © 2025 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Funding support and author disclosures The STRONG-HF trial was funded by Roche Diagnostics. Dr Myhre has received research grants from AstraZeneca and speaker and/or consulting fees from Amarin, AmGen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Novartis, Novo Nordisk, Orion Pharma, Pharmacosmos, Roche Diagnostics, Vifor, and Us2.ai. Dr Mebazaa has received grants from Roche Diagnostics, Abbott Laboratories, 4TEEN4, and Windtree Therapeutics; honoraria for lectures from Roche Diagnostics, Bayer, and MSD; is a consultant for Corteria Pharmaceuticals, S-form Pharma, FIRE-1, Implicity, 4TEEN4, and Adrenomed; and is a coinventor of a patent on combination therapy for patients having acute or persistent dyspnea. Drs Beth Davison, Edwards, Takagi, Barros, Novosadova, and Cotter are employees of Momentum Research, which has received grants for research from Abbott Laboratories, Amgen, Celyad, Cirius Therapeutics, Corteria Pharmaceuticals, Heart Initiative, Sanofi, Windtree Therapeutics, and XyloCor Therapeutics. Drs BD and Cotter are directors of Heart Initiative, a nonprofit organization. Dr Adamo has received speaker fees from Abbott Vascular and Medtronic. Dr Celutkiene has received personal fees from Novartis, AstraZeneca, Boehringer Ingelheim, Roche Diagnostics, and Pfizer. Dr Chioncel received grants from Servier. Dr Cohen-Solal has received honoraria for lectures or consultancy from AstraZeneca, Novartis, Vifor, Bayer, Merck, Sanofi, Abbott, and Boehringer Ingelheim. Dr Damasceno works for the Faculty of Medicine, Eduardo Mondlane University (Maputo, Mozambique), which received research grants from the Heart Initiative for their participation in this study. Dr Diaz has received supporting fees for coordination of STRONG-HF trial activities. Dr Filippatos has received lecture fees or was a committee member for trials and registries sponsored by Bayer, Vifor, Boehringer Ingelheim, Medtronic, Servier, and Amgen. Dr ter Maaten reports speaker and/or consultancy fees to institution from Novartis, Boehringer Ingelheim, Moderna, Roche, and Novo Nordisk, and receiving grants from Netherlands Heart Foundation, and Netherlands Organization for Scientific Research (NOW) outside the submitted work. Dr Metra has received personal fees from Amgen, Livanova, and Vifor Pharma as a member of executive committees of sponsored clinical trials and from AstraZeneca, Bayer, Boehringer Ingelheim, Edwards Lifesciences, and Roche Diagnostics for participation to advisory boards or for speaking at sponsored meetings. Dr Pagnesi has received personal fees from Abbott Laboratories, AstraZeneca, Boehringer Ingelheim, and Vifor Pharma. Dr Pang has received grants or research contracts from American Heart Association, Roche, Siemens, Ortho Diagnostics, Abbott, Beckman Coulter, and Siemens; consulting fees from Roche; honoraria from WebMD; and he has financial interest in The Heart Course. Dr Ponikowski reports grants and personal fees from Amgen, grants and personal fees from Servier, Boehringer Ingelheim, Vifor Pharma, Novartis, Bayer, Cibiem, AstraZeneca, BMS, Renal Guard Solutions, Impulse Dynamics, and Abbott Vascular, and personal fees from Berlin Chemie outside of the submitted work. Dr Sliwa has received grants from Medtronic, Servier, and Amylam and honoraria from MSD, Novartis, and Sanofi. Dr Tomasoni has received speaker fees from Boehringer Ingelheim, Alnylam Pharmaceuticals, and from Pfizer. Dr Voors has received consultancy fees or research support from AstraZeneca, Bayer, BMS, Boehringer Ingelheim, Cytokinetics, Myocardia, Merck, Novartis, Novo Nordisk, and Roche Diagnostics. Dr Lam is supported by a Clinician Scientist Award from the National Medical Research Council of Singapore; has received research support from Novo Nordisk and Roche Diagnostics; has served as consultant or on the Advisory Board/Steering Committee/Executive Committee for Alleviant Medical, Allysta Pharma, AnaCardio AB, Applied Therapeutics, AstraZeneca, Bayer, Boehringer Ingelheim, Boston Scientific, Bristol Myers Squibb, CardioRenal, CPC Clinical Research, Eli Lilly, Impulse Dynamics, Intellia Therapeutics, Ionis Pharmaceutical, Janssen Research & Development LLC, Medscape/WebMD Global LLC, Merck, Novartis, Novo Nordisk, Prosciento Inc, Quidel Corporation, Radcliffe Group Ltd, Recardio Inc, ReCor Medical, Roche Diagnostics, Sanofi, Siemens Healthcare Diagnostics, and Us2.ai; and serves as cofounder and nonexecutive director of Us2.ai. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Figures




References
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

