Adaptive Design for Phase II/III Platform Trial of Lassa Fever Therapeutics
- PMID: 39983682
- PMCID: PMC11845141
- DOI: 10.3201/eid3102.240251
Adaptive Design for Phase II/III Platform Trial of Lassa Fever Therapeutics
Abstract
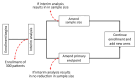
The current recommendation for treating Lassa fever with ribavirin is supported only by weak evidence. Given the persistent effects in areas with endemic transmission and epidemic potential, there is an urgent need to reassess ribavirin and investigate other potential therapeutic candidates; however, a robust clinical trial method adapted to Lassa fever epidemiology has not yet been established. We propose an adaptive phase II/III multicenter randomized controlled platform trial that uses a superiority framework with an equal allocation ratio and accounts for challenges selecting the primary end point and estimating the target sample size by using an interim analysis.
Keywords: Lassa fever; clinical trials; phase II/III multicenter randomized controlled platform trial; viruses.
Figures
References
-
- Nigeria Centre for Disease Control. Lassa fever situation report. Epi Week. 2023;52:2023.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources