Patient-derived monoclonal LGI1 autoantibodies elicit seizures, behavioral changes and brain MRI abnormalities in rodent models
- PMID: 39984135
- PMCID: PMC7618076
- DOI: 10.1016/j.bbi.2025.02.019
Patient-derived monoclonal LGI1 autoantibodies elicit seizures, behavioral changes and brain MRI abnormalities in rodent models
Abstract
Objective: Limbic encephalitis with leucine-rich glioma inactivated 1 (LGI1) protein autoantibodies is associated with cognitive impairment, psychiatric symptoms, and seizures, including faciobrachial dystonic seizures (FBDS). Patient-derived LGI1-autoantibodies cause isolated symptoms of memory deficits in mice and seizures in rats. Using a multimodal experimental approach, we set out to improve the validity of existing in vivo rodent models to further recapitulate the full clinical syndrome of anti-LGI1 antibody mediated disease.
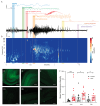
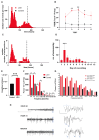
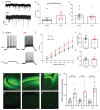
Methods: A monoclonal anti-LGI1 antibody (anti-LGI1 mAb) derived from a patient's CSF antibody-secreting cell was infused intracerebroventricularly (ICV) into rats and mice for one or two weeks, respectively. Cellular excitability of CA3 pyramidal neurons was determined in hippocampal slices. Structural changes in mouse brains were explored using MRI. Antibody effects on behavior and brain activity of rats were studied using video-EEG.
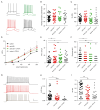
Results: Anti-LGI1 mAbs augmented the excitability of CA3 pyramidal neurons and elicited convulsive and non-convulsive spontaneous epileptic seizures in mice and rats. Mice displayed a hypoactive and anxious phenotype during behavioral testing. MRI revealed acutely increased hippocampal volume after ICV anti-LGI1 mAb infusion. Video-EEG recordings of juvenile rats uncovered two peaks of seizure frequency during the 7-day antibody infusion period resembling the natural progression of seizures in human anti-LGI1 encephalitis.
Interpretation: Our data strongly corroborate and extend our understanding of the direct pathogenic and epileptogenic role of human LGI1 autoantibodies.
Keywords: Animal model; Human monoclonal antibody; LGI1; Limbic encephalitis; Seizure.
Copyright © 2025. Published by Elsevier Inc.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

References
-
- Baudin P, Whitmarsh S, Cousyn L, Roussel D, Lecas S, Lehongre K, Charpier S, Mahon S, Navarro V. Kv1.1 channels inhibition in the rat motor cortex recapitulates seizures associated with anti-LGI1 encephalitis. Prog Neurobiol. 2022;213:102262. - PubMed
-
- Baumgartner T, Pitsch J, Olaciregui-Dague K, Hoppe C, Racz A, Rüber T, Becker A, von Wrede R, Surges R. Seizure underreporting in LGI1 and CASPR2 antibody encephalitis. Epilepsia. 2022;63:e100–e105. - PubMed
-
- Chabrol E, Navarro V, Provenzano G, Cohen I, Dinocourt C, Rivaud-Pechoux S, Fricker D, Baulac M, Miles R, Leguern E, Baulac S. Electroclinical characterization of epileptic seizures in leucine-rich, glioma-inactivated 1-deficient mice. Brain J Neurol. 2010;133:2749–2762. doi: 10.1093/brain/awq171. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

