The Medial Olivocochlear Efferent Pathway Potentiates Cochlear Amplification in Response to Hearing Loss
- PMID: 39984203
- PMCID: PMC11984096
- DOI: 10.1523/JNEUROSCI.2103-24.2025
The Medial Olivocochlear Efferent Pathway Potentiates Cochlear Amplification in Response to Hearing Loss
Abstract
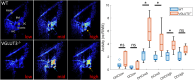
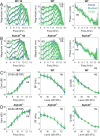
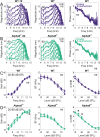
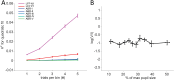
The mammalian cochlea receives efferent feedback from the brain. Many functions for this feedback have been hypothesized, including on short timescales, such as mediating attentional states, and long timescales, such as buffering acoustic trauma. Testing these hypotheses has been impeded by an inability to make direct measurements of efferent effects in awake animals. Here, we assessed the role of the medial olivocochlear (MOC) efferent nerve fibers on cochlear amplification by measuring organ of Corti vibratory responses to sound in both sexes of awake and anesthetized mice. We studied long-term effects by genetically ablating the efferents and/or afferents. Cochlear amplification increased with deafferentation using VGLUT3-/- mice, but only when the efferents were intact, associated with increased activity within OHCs and supporting cells. Removing both the afferents and the efferents using VGLUT3-/- Alpha9-/- mice did not cause this effect. To test for short-term effects, we recorded sound-evoked vibrations while using pupillometry to measure neuromodulatory brain state. We found no state dependence of cochlear amplification or of the auditory brainstem response. However, state dependence was apparent in the downstream inferior colliculus. Thus, MOC efferents upregulate cochlear amplification chronically with hearing loss, but not acutely with brain state fluctuations. This pathway may partially compensate for hearing loss while mediating associated symptoms, such as tinnitus and hyperacusis.
Keywords: brain state; cochlea; feedback; hearing; optical coherence tomography; outer hair cell; pupillometry.
Copyright © 2025 the authors.
Conflict of interest statement
J.S.O. and B.E.A. are founders of AO technologies, with the goal of translating inner ear imaging technologies for clinical purposes. The other authors declare no competing financial interests.
Figures









References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
