Striatal dopamine D2/D3 receptor regulation of human reward processing and behaviour
- PMID: 39984436
- PMCID: PMC11845780
- DOI: 10.1038/s41467-025-56663-7
Striatal dopamine D2/D3 receptor regulation of human reward processing and behaviour
Abstract
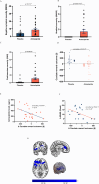
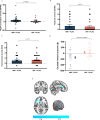
Signalling at dopamine D2/D3 receptors is thought to underlie motivated behaviour, pleasure experiences and emotional expression based on animal studies, but it is unclear if this is the case in humans or how this relates to neural processing of reward stimuli. Using a randomised, double-blind, placebo-controlled, crossover neuroimaging study, we show in healthy humans that sustained dopamine D2/D3 receptor antagonism for 7 days results in negative symptoms (impairments in motivated behaviour, hedonic experience, verbal and emotional expression) and that this is related to blunted striatal response to reward stimuli. In contrast, 7 days of partial D2/D3 agonism does not disrupt reward signalling, motivated behaviour or hedonic experience. Both D2/D3 antagonism and partial agonism induce motor impairments, which are not related to striatal reward response. These findings identify a central role for D2/D3 signalling and reward processing in the mechanism underlying motivated behaviour and emotional responses in humans, with implications for understanding neuropsychiatric disorders such as schizophrenia and Parkinson's disease.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: O.D.H. has received investigator-initiated research funding from and/or participated in advisory/speaker meetings organised by Angellini, Autifony, Biogen, Boehringer-Ingelheim, Eli Lilly, Elysium, Heptares, Global Medical Education, Invicro, Jansenn, Karuna, Lundbeck, Merck, Neurocrine, Ontrack/ Pangea, Otsuka, Sunovion, Recordati, Roche, Rovi and Viatris/Mylan. He was previously a part-time employee of Lundbeck A/v. Neither O.D.H. nor his family have holdings/a financial stake in any pharmaceutical company. M.B.W. is an employee of Perceptive Inc., London. T.R.M. is an employee and founder of Pasithea Therapeutics. R.A.M. has received speaker/consultancy fees from Boehringer Ingelheim, Janssen, Karuna, Lundbeck, Otsuka, and Viatris, and co-directs a company that designs digital resources to support the treatment of mental ill health. Other authors have reported no biomedical financial interests or potential conflicts of interest.
Figures

References
-
- Solmi M., et al. Incidence, prevalence, and global burden of schizophrenia—data, with critical appraisal, from the Global Burden of Disease (GBD) 2019. Mol. Psychiatry28, 5319–5327 10.1038/s41380-023-02138-4 (2023). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

