CLDN6 triggers NRF2-mediated ferroptosis through recruiting DLG1/PBK complex in breast cancer
- PMID: 39984471
- PMCID: PMC11845765
- DOI: 10.1038/s41419-025-07448-9
CLDN6 triggers NRF2-mediated ferroptosis through recruiting DLG1/PBK complex in breast cancer
Erratum in
-
Correction: CLDN6 triggers NRF2-mediated ferroptosis through recruiting DLG1/PBK complex in breast cancer.Cell Death Dis. 2025 Aug 21;16(1):632. doi: 10.1038/s41419-025-07910-8. Cell Death Dis. 2025. PMID: 40841418 Free PMC article. No abstract available.
Abstract
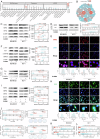
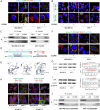
We previously identified CLDN6 as a pivotal tumor suppressor in breast cancer and unexpectedly discovered that overexpression of CLDN6 resulted in characteristic ultrastructural alterations of ferroptosis. However, the exact mechanism by which CLDN6 triggers ferroptosis is still elusive in breast cancer. Our study showed that CLDN6 was associated with ferroptosis in breast cancer patients. The integration of CLDN6 and ferroptosis demonstrated remarkable predictive prognostic performance. We observed that CLDN6 triggers NRF2-mediated ferroptosis in vitro and in vivo. Mechanistically, CLDN6 enhanced nuclear export of NRF2 by regulating the PBK-dependent AKT/GSK3β/FYN axis. Further CLDN6 recruited PBK to the cell membrane through the endosomal pathway and bound with the DLG1/PBK complex, thereby promoted the degradation of PBK by the UPS. This study elucidates the previously unrecognized mechanism of CLDN6 triggering NRF2-mediated ferroptosis through recruiting DLG1/PBK complex. This study provides a reliable biomarker for predicting prognosis and is anticipated to guide the selection of therapies targeting ferroptosis in breast cancer.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests. Ethics: The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Experimental Animal Ethical Committee of Jilin University (protocol code 2021.237 and 2022.72), the Ethics Committee of Shanghai Outdo Biotech CO and Hunan Aifang Biological (protocol code YB M-05-02).
Figures




References
-
- Sui SY, Xu SP, Pang D. Emerging role of ferroptosis in breast cancer: New dawn for overcoming tumor progression. Pharm Ther. 2022;232:27. - PubMed
-
- Zou Y, Xie J, Zheng S, Liu W, Tang Y, Tian W, et al. Leveraging diverse cell-death patterns to predict the prognosis and drug sensitivity of triple-negative breast cancer patients after surgery. Int J Surg (Lond, Engl). 2022;107:106936. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

