A short-term, high-caloric diet has prolonged effects on brain insulin action in men
- PMID: 39984682
- PMCID: PMC11946887
- DOI: 10.1038/s42255-025-01226-9
A short-term, high-caloric diet has prolonged effects on brain insulin action in men
Abstract
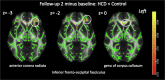
Brain insulin responsiveness is linked to long-term weight gain and unhealthy body fat distribution. Here we show that short-term overeating with calorie-rich sweet and fatty foods triggers liver fat accumulation and disrupted brain insulin action that outlasted the time-frame of its consumption in healthy weight men. Hence, brain response to insulin can adapt to short-term changes in diet before weight gain and may facilitate the development of obesity and associated diseases.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: M.H. reports research grants from Boehringer Ingelheim and Sanofi to the University Hospital of Tübingen; participation in advisory board for Boehringer Ingelheim, Sanofi and Amryt; and lecture fees from Amryt, Novartis, Sanofi, Eli Lilly, Novo Nordisk and Boehringer Ingelheim. The other authors declare no competing interests.
Figures
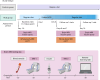
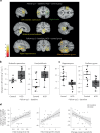
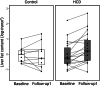
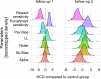
References
-
- DeFronzo, R. A. et al. Type 2 diabetes mellitus. Nat. Rev. Dis. Primers1, 15019 (2015). - PubMed
-
- Kullmann, S. et al. Central nervous pathways of insulin action in the control of metabolism and food intake. Lancet Diabetes Endocrinol.8, 524–534 (2020). - PubMed
-
- Hallschmid, M. Intranasal insulin. J. Neuroendocrinol.33, e12934 (2021). - PubMed
-
- Scherer, T., Sakamoto, K. & Buettner, C. Brain insulin signalling in metabolic homeostasis and disease. Nat. Rev. Endocrinol.17, 468–483 (2021). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

