Relapse-free survival in a pediatric patient with recurrent EZH2-mutant melanoma treated with adjuvant tazemetostat
- PMID: 39984702
- PMCID: PMC11845573
- DOI: 10.1038/s41698-025-00826-8
Relapse-free survival in a pediatric patient with recurrent EZH2-mutant melanoma treated with adjuvant tazemetostat
Abstract
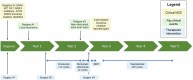
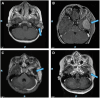
Enhancer of zeste homolog 2 (EZH2) is an essential epigenetic regulator of H3K27 histone methylation and is mutated or overexpressed in a wide variety of cancers. In melanoma, EZH2 overexpression contributes to excessive trimethylation of H3K27 on tumor suppressor genes and has been proposed to be a mechanism of tumor progression and metastasis. EZH2-targeted therapies have been successfully used to treat patients with follicular lymphoma and epithelioid sarcoma, but their clinical use in melanoma has not been described. Here, we describe a pediatric patient with multiply relapsed melanoma harboring an EZH2 A692V missense mutation, treated adjuvantly with the EZH2 inhibitor tazemetostat, who experienced a prolonged relapse-free survival.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: C.A.P. has received research grants from Kura Oncology and Novartis Institutes for Biomedical Research (unrelated to this manuscript) and consulting fees from Day One Therapeutics (unrelated). Related to this manuscript, E.J.L. has received support from the Bloomberg~Kimmel Institute for Cancer Immunotherapy, the Marilyn and Michael Glosserman Fund for Basal Cell Carcinoma and Melanoma Research, the Barney Family Foundation, and the Laverna Hahn Charitable Trust. E.J.L is a consultant/advisor for Agenus, Bristol-Myers Squibb, CareDx, Eisai, Genentech, HUYA Bioscience International, Immunocore, Instil Bio, Lyvgen, Merck, Merck KGaA, Natera, Nektar, Novartis, OncoSec, Pfizer, Rain Therapeutics, Regeneron, Replimune, Sanofi-Aventis, Sun Pharma, Syneos Health (unrelated). E.J.L. has institutional research funding from Bristol-Myers Squibb, Haystack Oncology, Merck, Regeneron, Sanofi (unrelated). EJ.L holds stock in Iovance Biotherapeutics (unrelated). The other co-authors declare that they have no competing interests.
Figures
References
-
- Gounder, M. et al. Tazemetostat in advanced epithelioid sarcoma with loss of INI1/SMARCB1: an international, open-label, phase 2 basket study. Lancet Oncol.21, 1423–1432 (2020). - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

