Alzheimer disease-associated tau post-translational modification mimics impact tau propagation and uptake
- PMID: 39984820
- PMCID: PMC12096005
- DOI: 10.1093/jnen/nlaf007
Alzheimer disease-associated tau post-translational modification mimics impact tau propagation and uptake
Abstract
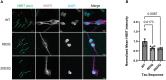
As Alzheimer disease (AD) progresses, pathological tau spreads by cell-to-cell propagation of tau. This study aims to elucidate the impact of AD-associated post-translational modifications of tau-on-tau propagation. Tau propagation reporter constructs distinguishing donor cells from recipient cells were developed, and additional constructs were made with tau residues mutated from serine or threonine to aspartate to mimic the negative charge of a phosphorylation and/or from lysine to glutamine to mimic the charge-neutralizing effect of acetylation. Flow cytometry was used to quantify donor and recipient cells. This revealed that the mutations generally tended to reduce tau propagation compared to wildtype tau. Recombinant tau containing either wildtype or posttranslational modification mimicking mutations were used to treat Chinese hamster ovary cells or human induced pluripotent stem cell-derived neurons to quantify tau uptake, revealing that the mutations generally resulted in reduced uptake compared to wildtype tau. Surface plasmon resonance revealed that the mutations had a reduced affinity for lipoprotein receptor-related protein 1 (LRP1), a tau uptake receptor, compared to wildtype tau. Overall, these results suggest that AD-associated posttranslational modification mimicking mutations reduce the cell-to-cell propagation of tau by reducing tau uptake by recipient cells, which may be in part due to reduced binding affinity to LRP1.
Keywords: Alzheimer disease; acetylation; low-density lipoprotein receptor-related protein 1; phosphorylation; post-translational modification; propagation; tau.
© The Author(s) 2025. Published by Oxford University Press on behalf of American Association of Neuropathologists, Inc.
Conflict of interest statement
J.R.D. reports prior consulting for I-Mab Biopharma. B.T.H. owns stock in Novartis; he serves on the scientific advisory board of Dewpoint and has an option for stock. He serves on a scientific advisory board or is a consultant for AbbVie, Alexion, Ambagon, Aprinoia Therapeutics, Arvinas, Avrobio, AstraZenica, Biogen, BMS, Cure Alz Fund, Cell Signaling, Dewpoint, Latus, Novartis, Pfizer, Sanofi, Sofinnova, Vigil, Violet, Voyager, WaveBreak. His laboratory is supported by research grants from the National Institutes of Health, Cure Alzheimer’s Fund, Tau Consortium, and the JPB Foundation—and a sponsored research agreement from Abbvie.
Figures





References
MeSH terms
Substances
Grants and funding
- Alzheimer's Association and Cure Alzheimer's Fund
- R01 AG073236/AG/NIA NIH HHS/United States
- T32 HL007698/HL/NHLBI NIH HHS/United States
- P30 AG062421/AG/NIA NIH HHS/United States
- T32HL007698/GF/NIH HHS/United States
- AARF-22-924352/Alzheimer's Association Research
- Jack Satter Foundation
- S10 OD030379/OD/NIH HHS/United States
- NIH P30AG062421/Jack Satter Foundation
- R35HL135743/GF/NIH HHS/United States
- R35 HL135743/HL/NHLBI NIH HHS/United States
- S10OD030379/GF/NIH HHS/United States
- S10OD00379/Surface plasmon resonance
- AARF-22-924352/Alzheimer's Association Research Fellowship
- 5K08AG078341/GF/NIH HHS/United States
- 2018-AACSF-592307/Alzheimer's Association Clinician Scientist
- DP1 OD000379/OD/NIH HHS/United States
- K08 AG078341/AG/NIA NIH HHS/United States
- R21 AG083236/AG/NIA NIH HHS/United States
- RF1AG083236/GF/NIH HHS/United States
- RF1 AG073236/AG/NIA NIH HHS/United States
- JPB Foundation
- P30AG062421/GF/NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

