FTO inhibition mitigates high-fat diet-induced metabolic disturbances and cognitive decline in SAMP8 mice
- PMID: 39984825
- PMCID: PMC11843768
- DOI: 10.1186/s10020-025-01126-4
FTO inhibition mitigates high-fat diet-induced metabolic disturbances and cognitive decline in SAMP8 mice
Abstract
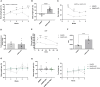
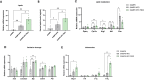
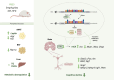
This study investigated the effects of fat mass and obesity-associated (FTO) inhibition on cognitive function and metabolic parameters of senescence-accelerated mouse prone 8 (SAMP8) mice fed a high-fat diet (HFD). SAMP8 mice fed an HFD exhibited increased body weight, impaired glucose tolerance, and elevated serum leptin levels. In epididymal white adipose tissue (eWAT), pharmacological treatment with FB23, a well-established FTO inhibitor, increased leptin production and modulated genes involved in lipid metabolism (Cpt1a, Atgl, Hsl, Fas), oxidative stress (OS) (Bip, Edem), and inflammation (Mcp1, Tnfα). Expression of hepatic genes related to lipid metabolism (Cpt1a, Atgl, Mgl, Dgat2, Srebp, Plin2) and OS (catalase, Edem) were modulated by FB23, although hepatic steatosis remained unchanged. Remarkably, FB23 treatment increased m6A RNA methylation in the brain, accompanied by changes in N6-methyladenosine (m6A)-regulatory enzymes and modulation of neuroinflammatory markers (Il6, Mcp1, iNOS). FTO inhibition reduced the activity of matrix metalloproteases (Mmp2, Mmp9) and altered IGF1 signaling (Igf1, Pten). Notably, enhanced leptin signaling was observed through increased expression of immediate early genes (Arc, Fos) and the transcription factor Stat3. Improved synaptic plasticity was evident, as shown by increased levels of neurotrophic factors (Bdnf, Ngf) and restored neurite length and spine density. Consistent with these findings, behavioral tests demonstrated that FB23 treatment effectively rescued cognitive impairments in SAMP8 HFD mice. The novel object recognition test (NORT) and object location test (OLT) revealed that treated mice exhibited enhanced short- and long-term memory and spatial memory compared to the HFD control group. Additionally, the open field test showed a reduction in anxiety-like behavior after treatment with FB23. In conclusion, pharmacological FTO inhibition ameliorated HFD-induced metabolic disturbances and cognitive decline in SAMP8 mice. These results suggest that targeting FTO may be a promising therapeutic approach to counteract obesity-induced cognitive impairment and age-related neurodegeneration.
Keywords: Aging; Epigenetics; FTO; Metabolic disorders; Neurodegenerative disease; m6A.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All procedures involving animals, including behavioral testing and dissection and removal of brains, followed ARRIVE and the standard ethical guidelines (Council of the European Communities Directive 2010/63/EU and Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research, National Research Council 2003) and were approved by the Institutional Animal Care and Generalitat de Catalunya (#10291, January 28, 2018). Every effort was made to minimize the number of mice used and their suffering. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures







References
-
- Amidfar M, Askari G, Kim YK. Association of metabolic dysfunction with cognitive decline and Alzheimer’s disease: a review of metabolomic evidence. Prog Neuropsychopharmacol Biol Psychiatry. 2024;128:110848. - PubMed
-
- Berlivet S, Scutenaire J, Deragon JM, Bousquet-Antonelli C. Readers of the m 6 A epitranscriptomic code. Biochim Et Biophys Acta Gene Regul Mech. 2019;1862:329–42. - PubMed
MeSH terms
Substances
Grants and funding
- PID2021-122116OB-100/Ministerio de Economía, Industria Economía, Industria y Competitividad (Agencia Estatal de Investigación, AEI) and European Union NextGenerationEU/PRTR
- PDC2022-133441-I00/Ministerio de Economía, Industria Economía, Industria y Competitividad (Agencia Estatal de Investigación, AEI) and European Union NextGenerationEU/PRTR
- PID2020-114953RB-C21/Ministerio de Economía, Industria Economía, Industria y Competitividad (Agencia Estatal de Investigación, AEI) and European Union NextGenerationEU/PRTR
- PID2022-139016OA-I00/Ministerio de Economía, Industria Economía, Industria y Competitividad (Agencia Estatal de Investigación, AEI) and European Union NextGenerationEU/PRTR
- CIBERDEM/CIBER de Diabetes y Enfermedades Metabólicas Asociadas (CIBERDEM)
- 2021 SGR 00357/the Government of Catalonia
- CB06/03/0001/CIBEROBN
- 2021SGR00367/Carlos III Health Institute project and the Government of Catalonia
- Producte 0092/Departament d'Empresa i Coneixement de la Generalitat de Catalunya 2023
- Llavor 005 and 007/Departament d'Empresa i Coneixement de la Generalitat de Catalunya 2023
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

